Abstract
Microwave ablation (MWA) is an alternative locoregional therapy to surgical resection of solid tumors in the treatment of malignancies, and is widely used for hepatic tumors. It has a slightly higher overall survival (OS) rate compared to external beam radiation therapy (EBRT), and proton beam therapy (PBT), and better long-term recurrence-free OS rate compared to radiofrequency ablation (RFA). In this paper, current commercial devices, most recent noncommercial designs, and the principles behind them alongside the recently reported developments and issues of MWA are reviewed. The paper also provides microscopic insights on effects of microwave irradiation in the body. Our review shows that MWA is a safe and effective, minimally invasive method with high ablation completion rates. However, for large tumors, the completion rates slightly decrease, and recurrences increase. Thus, for large tumors we suggest using a cooled shaft antenna or multiple antenna placements. Comparisons of the two common ablation frequencies 915 MHz and 2.45 GHz have shown inconsistent results due to non-identical conditions. This review suggests that 915 MHz devices are more effective for ablating large tumors and the theory behind MWA effects corroborates this proposition. However, for small tumors or tumors adjacent to vital organs, 2.45 GHz is suggested due to its more localized ablation zone. Among the antenna designs, the double-slot antenna with a metallic choke seems to be more effective by localizing the radiation around the tip of the antenna, while also preventing backward radiation towards the skin. The review also pertains to the use of MWA in COVID-19 patients and risk factors associated with the disease. MWA should be considered for COVID-19 patients with hepatic tumors as a fast treatment with a short recovery time. As liver injury is also a risk due to COVID-19, it is recommended to apply liver function tests to monitor abnormal levels in alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and other liver function indicators.
Keywords: Microwave, Ablation, Percutaneous, Liver, Tumor, Hepatocellular carcinoma, COVID-19
Microwave, ablation, percutaneous, liver, tumor, hepatocellular carcinoma, COVID-19
1. Introduction
Tumor ablation involves the removal or destruction of tumors by means of thermal therapies [1, 2]. Among different ablation techniques, Microwave ablation (MWA) is capable of being used as a minimally invasive technique and has gained attention in 21st century, along with advances in imaging technology [2, 3, 4, 5, 6, 7]. The current work is a comprehensive and unique review article about percutaneous hepatic MWA that covers the science behind the technique, the engineering of the microwave delivery to the tissue, commercial devices, case reports after 2014 for MWA of different tumor sizes and ablation devices, limitations of this technique, new insights from the analysis of the literature reviewed, as well as the effect of the current pandemic on MWA. We believe MWA can be important these days with the progression of the COVID-19 pandemic [8, 9], for reasons discussed in this review. Recent data has also shown that the use of MWA has increased compared to surgical resection during the pandemic, primarily due to shorter postoperative stay and hospitalization time following MWA [9, 10].
MWA ablation techniques can use one or multiple antennas to deliver microwave radiations to the tissue. The antennas used in MWA are commonly thin and can be placed on or inserted into the tumor tissue. In modern MWA devices, antennas up to 2 mm in diameter can deliver a high-power microwave radiation. The ease of use and small width of the antenna make it a suitable candidate for percutaneous use.
Microwave radiation in general has applications in various fields such as communications [11], medicine [12, 13], food industry [13, 14], and manufacturing [15]. However, in medicine, one of the main applications of microwave radiation is as a heat source and a means of delivering powerful energy to parts of human body such as in [16]:
-
1.
Cardiology
-
2.
Benign prostatic hyperplasia (BPH)
-
3.
Tumor ablation
-
4.
Endometrial ablation
-
5.
Liposuction
-
6.
Microwave balloon angioplasty
-
7.
Microwave treatment of microbial infections [13]. This is mostly focused on two types of medical applications of microwave radiation: killing or inactivating pathogenic microbes (e.g., some bacteria), and the SARS-CoV-2 virus [13].
This review article is focused on liver disease and in particular, liver cancer. Among cancers, liver cancer has been classified as one of the leading causes of cancer related deaths, with the 5th lethal level in the United States [17, 18]. Liver cancer occurring in liver cells (hepatocytes) is referred to as hepatocellular carcinoma (HCC). This type of cancer is the most common type of primary liver cancers, causing about 80% of hepatic malignancies globally, followed by cholangiocarcinoma (CC), which is the cancer of the bile ducts, that are responsible for carrying bile to the gallbladder. A major cause of HCC is chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections [19], as insertion of the viral hepatitis B genome into the telomerase reverse transcriptase (TERT) of the human genome results in a mutation accounting for 60% of HCC cases [20]. HVB and HCV are more prevalent in Asia and Africa, and less prevalent in developed countries. However, HCV is slightly more prevalent than HBV in developed countries and for instance, HCV rates in Canada and the United States are 0.8% and 1.8%, respectively [21, 22, 23, 24]. The significance of liver diseases can also be perceived by the fact that the Nobel Prize in Physiology or Medicine 2020 was awarded for the discovery of HCV as an unknown cause of chronic hepatitis that causes cirrhosis and liver cancer.
The histopathological features of HCC include vascularized tumors with cytological atypia, mitotic activity, Kupffer cell count reduction, and reticulin network disassembly [25]. However, these factors tend to be patient dependent and will manifest differently from person to person [26]. CC arises in any segment of the intrahepatic (pre)biliary tree, and due to its proximity to the liver, it is an increased risk factor for metastatic CC of the liver [27]. Following MWA of the liver, the amount of necrosis of the ablated area is dependent on the irradiated power used [28]. The ablation area is confined by palisading, histiocytic, giant cells, and accompanying inflammation [29]. Ablated area has faded nuclei and eosinophilic cytoplasm [29]. In areas where ablation could not completely destroy malignant tissue, tumor cells display cytoplasmic eosinophilia, but with present nuclei, indicating these tumor cells could still be functional [29]. The majority of MWA lesions develop a surrounding rim of characteristic tissues, which takes approximately a week to heal/disappear. Increased disappearance time or failure for the ring to fade could be an indicator of residual tumor tissue or tumor reoccurrence [28, 29]. Post treatment biopsies 1–3 months following successful MWA of all malignant tissues reveals that tumor tissue will be completely replaced by fibrosis [28].
One of the most common applications of microwave ablation is for the treatment of liver cancer. However, microwave ablation applications in the liver are not limited to cancer therapy, and includes the ablation of liver cysts as well [30]. Microwave ablation of liver tissue is the consequence of localized microwave heating with an antenna, which leads to cell destruction and shrinkage of the tissue. Ex vivo microwave heating of bovine liver for the purpose of tissue ablation has shown to shrink the tissue about 30% [31]. The conventional liver cancer treatment is surgical resection, which may not always be an option for most patients, due to limitations such as the presence of cirrhosis and poor liver function [32, 33, 34]. Other methods such as liver transplants and radiation therapy have their own well-known complications. Moreover, MWA ablation provides an alternative minimally invasive and safe method to the surgical resection, as described in section 4 [35].
Thermal ablation techniques such as radiofrequency ablation (RFA) and microwave ablation (MWA) are two alternatives to surgical resection [36, 37]. There are other alternative techniques, such as irreversible electroporation, cryoablation, high intensity focused ultrasound (HIFU), and percutaneous ethanol injection (PEI). Among ablation methods, MWA is ideal for treatment of vascularized tissues and tumors adjacent to blood vessels, since blood perfusion has less of a negative effect on microwave thermal ablation compared to RF ablation [38, 39, 40]. In fact, in RFA, convective heat transfer will occur between the hot tissue and the colder blood flowing through the tissue. Since RFA growth is slow, the blood perfusion acts as a heatsink and prevents the ablation process especially when large vessels with high blood perfusion rates are in the vicinity [41]. Moreover, MWA has shown to have a sharper effective zone boundary compared to techniques such as RFA, cryoablation (CRYO) and irreversible electroporation (IRE) [42]. In a study on HCC patients treated with MWA and RFA, MWA had similar 1-year overall survival (OS) rate to that of RFA, but showed a better 5-year OS without recurrence (28.1% vs 19.6%) [43].
Some other non-thermal therapy techniques, such as external beam radiation therapy (EBRT) and proton beam therapy (PBT), use ionizing radiation [44, 45, 46]. They can be effective treatments, however, as a comparison, the average OS rate after 1 year follow up for 770 HCC patients treated with MWA from the data in this review (from small to large tumors) was 88% [47, 48, 49, 50]; whereas the average 1-year OS rate for EBRT among 1051 patients was 83% [45]. In a study on 162 HCC patients treated with PBT, the average 1-year OS rate was 79% [51]. So overall, MWA seems to be slightly more effective than other types of radiation therapy.
2. Technical discussion
MWA is done by irradiating the tumor using the microwave radiation and typically, deposition of the microwave induced heat on demand in the tumor, which leads to increase in the temperature of the tissue, therefore killing the tumor cells. In microwave ablation, the amount of absorbed microwave energy by the tissue depends on its permittivity. The permittivity is frequency-dependent and describes how the material behaves in the presence of an external electric field. When a tissue as a dielectric medium is exposed to an external electric field, the organization of the electric charges will be affected by the external electric field via electric dipole reorientation of its polar molecules and charge migration of its ions. Other effects are the changes to the hydrogen bonded clusters and network of the hydrogen bonding. In biological systems like the body, other factors also play role, such as changes to the hydrophobic and hydrophilic structures. As the electric field moves through the dielectric, it shifts the electrons alongside the field direction in such a way that the negative charges are attracted to the positive direction of field, and vice versa, to reach an equilibrium. The shift is to the partial charges in the medium. This shift creates an opposite field which cancels out that electric field. Higher permittivity means more shift in electrons, and in general, partial charges which results in higher microwave energy deposition. Some of this deposited energy is then dissipated into heat and rises the tissue temperature.
Table 1 demonstrates the difference between the permittivity of normal and malignant human liver tissue at two common microwave frequencies. There is a difference between the electrical properties of normal and malignant cells, which results in higher microwave energy dissipation in the tumor and higher temperatures. The difference has been associated to an increase in negative electrical charge on the outer surface of the cellular membranes when transforming to malignant cells [52], which consequently affects the transmembrane potential [53]. The increased negative charge results in higher electrical conductivity of the tissue and an increase in migration of the mobile charges under an applied external electric field. The increase in temperature is due to the dissipation of this excess ordered kinetic energy by coupling of this motion with other degrees of freedom in the tissues. This energy dissipation is the cause of the increased temperature. Despite this proposed mechanism, these effects are not fully understood and need extensive research to probe all microwave effects in the normal and malignant tissues. Such an understanding requires the following investigations: 1) impacts of the electric fields of the microwave independent of thermal effects, if any; 2) impacts of the magnetic fields of the microwave independent of thermal effects, if any; 3) impacts of the thermal effects of the microwave; 4) probing such impacts using in situ methods [12].
Table 1.
Average relative permittivity and effective conductivity for in vivo normal and malignant human liver tissue at 915 MHz and 2.45 GHz [54].
| Frequency | Normal |
Malignant |
||
|---|---|---|---|---|
| 915 MHz | 59.94 | 1.16 | 64.09 | 1.34 |
| 2.45 GHz | 57.55 | 1.95 | 62.44 | 2.18 |
The dielectric properties of materials in general are commonly temperature dependent. In a study [55], a relation for relative permittivity of the liver tissue at 2.45 GHz and at different temperatures ranging from 1 °C to 100 °C was evaluated.
| (1) |
where is the temperature of the tissue in °C. Although the value is slightly different with that of shown in Table 1, it implies that the relative permittivity does not vary much in the mentioned temperature range.
2.1. Microwave ablation devices
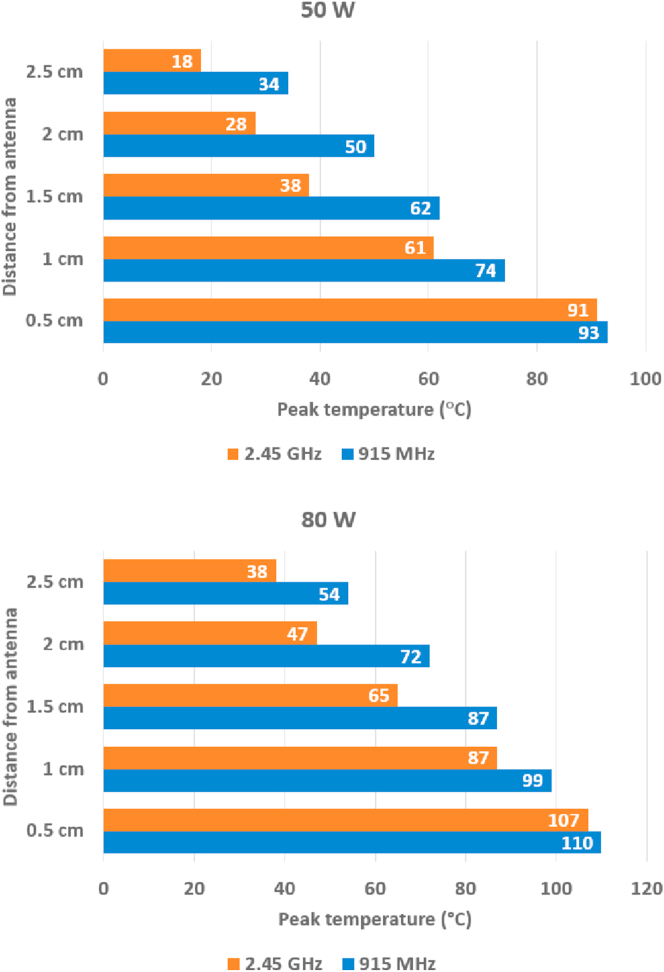
What we describe in this section is not limited to liver cancer. It can apply to other liver diseases and to infectious diseases that can be treated using microwave systems [15]. Common MWA systems are divided into three main parts: the microwave generator, the coaxial cable, and the microwave antenna [56]. The microwave generator could be a solid-state device or a magnetron. The two main frequencies provided by microwave sources for microwave ablation are 915 MHz and 2.45 GHz [57]. In a study on ex vivo porcine liver using a MWA system manufactured by Kangyou Medical using a cooled-shaft antenna, it was shown that the peak temperature of the tissue at distances greater than 1 cm at 915 MHz was significantly higher than that at 2.45 GHz [58]. According to the authors, this is due to the higher penetration depth and lower attenuation of 915 MHz microwave radiation compared to those of 2.45 GHz Figure 1 demonstrates the comparison of the peak temperature achieved at the two frequencies, at two different powers 50 W and 80 W, and at different distances from the cool-shaft antenna based on their result. This result conforms to the permittivity values in Table 1.
Figure 1.
The comparison of peak temperature achieved for ex vivo MWA of porcine liver at the two common frequencies 915 MHz and 2.45 GHz, at two different powers 50 W and 80 W, and at different distances from a cooled shaft antenna [58].
On the other hand, in another study, the difference of the two frequencies were evaluated on 48 patients with a total of 124 hepatic tumors and it was shown that a 2.45 GHz applicator provides a larger ablation zone and significantly shorter ablation time compared to a 915 GHz, making the 2.45 GHz frequency more suitable for larger tumors [59]. However, the 915 MHz system had three separate 45 W antennas, whereas the 2.45 GHz system had a single 100 W antenna, which makes the comparison difficult. The reason for more effectiveness of 2.45 GHz frequency is most probably the impedance mismatch between cables and antennas in the 915 MHz system [59].
Considering this inconsistency, the differences in the thermal ablation by microwave radiation with the two frequencies were investigated with three approaches: theoretical, simulation, and ex vivo experiment [60]. The ex vivo experiment was performed by using a custom-designed, single and dual interstitial dipole non-cooled antenna fed with a 30 W microwave source and with effort to minimize the differences of any other factors between the two frequencies. For an infinitesimal dipole antenna, the E- and H-field components in spherical coordinates are [61]:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
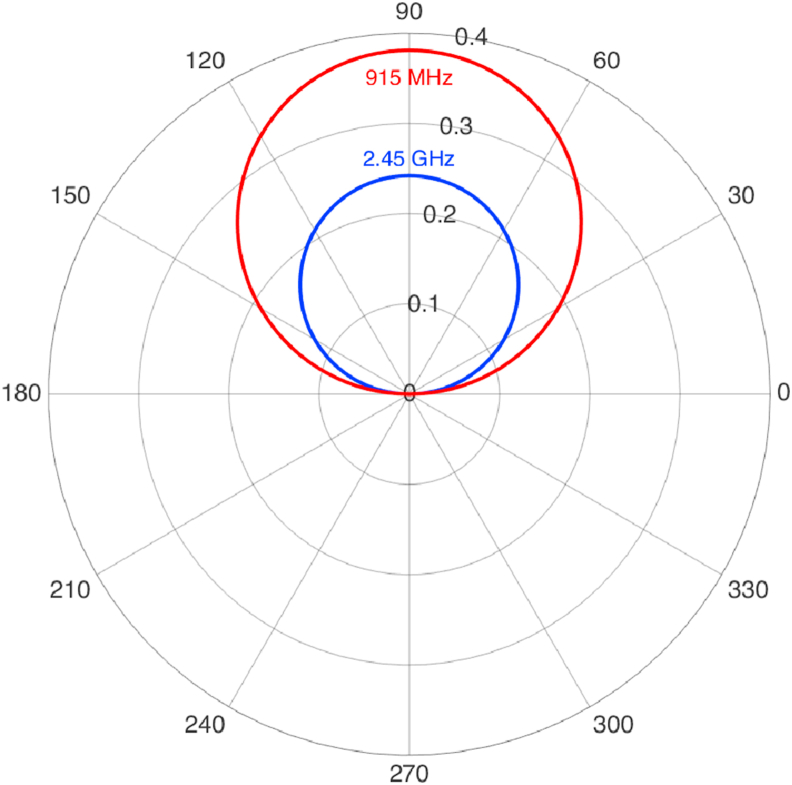
where is the total length of the dipole antenna, is the current amplitude, is the wave number, and are the electric and magnetic fields respectively, and is the wave impedance which is dependent of the permeability and the permittivity of the medium. is the wavenumber and where is the wavelength. Figure 2 is the illustration of the wave propagation for an infinitesimal dipole antenna at 2.45 GHz and 915 MHz based on Eqs. (4) and (5).
Figure 2.
The wave pattern for the electric component of an infinitesimal dipole antenna at 915 MHz and 2.45 GHz with an arbitrary constant current amplitude for both frequencies.
Both simulations and experimental results of a single antenna show that 915 MHz provides a wider spherical ablation zone compared to 2.45 GHz. At large distances from the antenna, using 915 MHz will result in reaching higher temperatures in shorter times. Whereas for small distances from the antenna, 2.45 GHz is more effective by reaching higher temperatures faster. This is due to higher ion displacement frictions by the microwave radiation, which results in more absorption near the antenna and attenuation for 2.45 GHz compared to 915 MHz. The frequencies are not limited to 2.45 GHz and 915 MHz. In fact, a recent study has shown that with a novel 14.5 GHz MWA system for ex vivo ablating of human liver, an efficient, reproducible, and large spherical ablation zone can be achieved [62]. It has also been identified as the optimum frequency for ablating tissues with the high-power microwave radiation and it provides a limited depth of penetration that leads to a faster and more sharp-edged spherical ablation zone. This microwave energy is dissipated in the tumor. This will also result in performing ablation in a shorter time. The applied MicroBlate system in this study has a solid-state microwave source with maximum output of 50 W, a coaxial antenna with a ceramic sheath, and a low power mode (10 mW) for measuring the complex impedance of the tissue [63]. A new multi-functional antenna design capable of working at this frequency has been introduced [64]. The permittivity of the liver at this frequency is half of the reported ones for 915 MHz and 2.45 GHz [65]. The depth of electric penetration in the tissue is the depth that the electric field reaches to 1e times that of at antenna and is defined by:
| (7) |
where is the electric field penetration depth, and are absolute permittivity and absolute permeability of the tissue respectively, is the frequency of the microwave radiation, and is the conductivity of the tissue. For example, according to this formula, the electric penetration depth in colon at 2.45 GHz and 14.5 GHz are 19.3 mm and 1.83 mm respectively [63].
2.2. Antenna
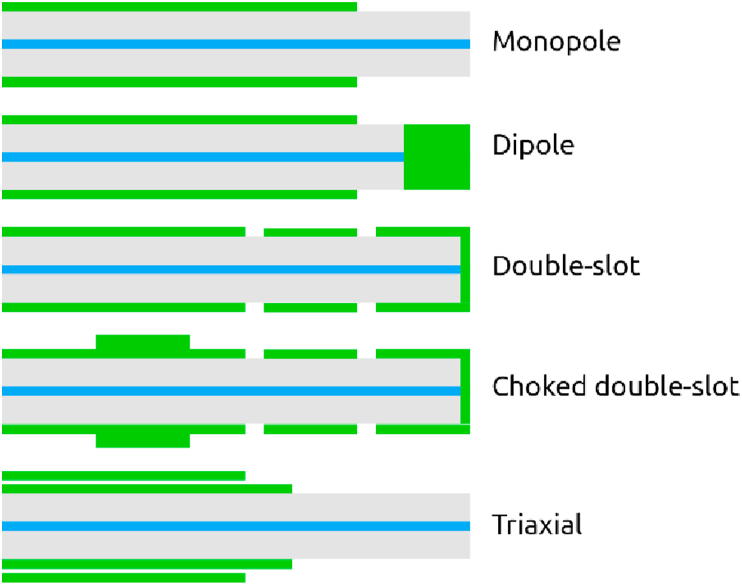
The main part of the microwave delivery is the antenna. The radiation pattern inside the tissue and the reflection coefficient are the parameters that are related to the antenna design [66]. For minimally invasive purposes and ease of use, microwave radiation is commonly delivered using a microwave coaxial antenna (MCA). The most common microwave delivery systems for microwave ablation are coaxial-based systems (Figure 3) which typically use one of these three antennas [57]:
-
1.
Dipole antenna
-
2.
Monopole antenna
-
3.
Slot antenna
Figure 3.
Schematic representation of the common antenna designs.
And the three shapes of antennas (Figure 3) are as follows [67]:
-
1.
Straight
-
2.
Loop
-
3.
Triaxial
Dipole antenna is the simplest form of antenna which comprises of two axial conductive components. The current oscillations generate the microwave radiation. Monopole antenna is like a dipole antenna, but a ground plate is used instead of one the conductive components. Slot coaxial antennas are currently the most popular ones for MWA applications due to their small size and low manufacturing costs [55]. They comprise of a radiating element which is made by cutting an opening on a ground plane [61]. This could be single-slot or double-slot. The latter one provides a more localized and spherical microwave absorption pattern in the tissue.
In slot coaxial antennas, with impedance mismatch between the antenna and the tissue, propagation of surface currents generated at the slot between the outer conductor of the coaxial cable and the catheter may cause an extreme increase in the temperature at the electric current site and cause the backward heating problem. Sometimes, backward heating is useful, such as to stop residual bleeding [67], but for minimally-invasive methods and in particular, percutaneous methods, it may cause some undesired burns to the skin around the insertion area. Although a simple solution is cooling the insertion area, a modification to the slot antenna design could prevent or minimize this problem, such as the coaxial choke and extended tip choke designs [68]. The straight antenna design is the simplest shape of antenna which is widely used whereas the loop antenna comprises a round loop-shaped antenna which is more suitable for ablating large surface tumors [69]. The triaxial antenna will be discussed later in details in this article. Figure 3 demonstrates the schematic representation of the common antenna designs.
2.3. Choked antenna
The choked design of slot antennas was originally designed for cardiac MWA, but can be used in percutaneous hepatic MWA applications as well [70]. They are used as a solution to the backward heating problem, by connecting an annular thin metallic choke, usually of the wavelength long to minimize the reflected microwave power towards the whole antenna shaft and to localize the microwave power in the distal tip of the antenna [71]. In fact, the choke matches the antenna to the coaxial transmission line and the proximal end of the choke acts as an open circuit to prevent any returned current from transmission line. In addition, the capacitance of the tip increases due to the enlarged inner conductor and the annular cap, leading to enhancement of microwave radiation emitted from the tip of the antenna [69].
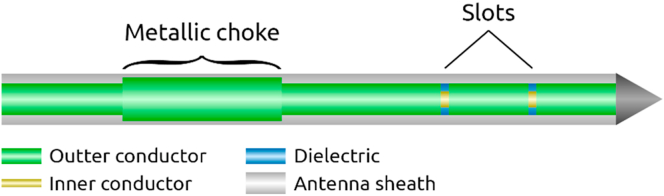
Although this type of antenna has low reflection and good microwave absorption pattern inside the tissue, the tip needs to be inserted up to 1.5 mm larger in diameter than the feed cable, since the surrounding metallic choke around the antenna causes an increase in diameter [67]. Figure 4 demonstrates a double-slot antenna with the metallic choke.
Figure 4.
Axial and radial schematics of a coaxial-based double slot choked antenna designed for hepatic microwave ablation (MWA).
In a study, some designs of this type of antenna have been discussed and have optimized the position of the metallic choke by using finite element method (FEM) electromagnetic simulations to achieve the minimum power reflection coefficient of -20.9 dB and minimized the backward heating [72]. This increases the overall MWA efficiency and leads to a better distribution of microwave energy in the liver in a larger ablation zone and decrease in undesired microwave energy along the antenna shaft.
2.4. Triaxial antenna
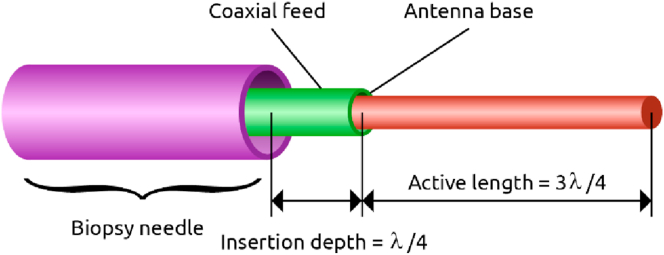
Triaxial antenna is a new microwave antenna design for ablation, which has been optimized for delivering a good microwave absorption pattern (i.e., more spherical pattern and low power reflection) with keeping the insertion depth at a minimum [73, 74]. This antenna is a coaxial monopole antenna within a biopsy needle [75]. The biopsy needle creates a triaxial structure when inserted into the tissue and maximizes the energy transfer to the tissue while minimizing the heating of the feed cable and invasiveness [73]. Figure 5 shows a schematic representation of the antenna.
Figure 5.
Schematic representation of a coaxial monopole antenna inserted through a biopsy needle. The active length of the antenna and the insertion depth through the needle can be adjusted according to the tissue properties.
2.5. Coaxial cable
As previously mentioned, coaxial cable is the conventional delivery method of microwave power from the source to the antenna. The attenuation constant is a measure of power loss in the cable which has two components [16]:
| (8) |
where is related to conductor loss and is related to dielectric loss. The dielectric loss is a measure of how much electromagnetic energy dissipates when the waves propagate inside a material due to the relaxation and resonance effects of the dipole molecules. The relaxation effect occurs when the electric field of the microwave radiation polarizes the dielectric material dipole molecules absorb and re-scatter the electric field. But the dipole response is not instantaneous and is always delayed by some phase with respect to the field and it opposes to the electric field in each cycle. The resonance effect happens when the electric field of the microwave radiation resonates with the movement of partial charges or and vibrionic/rotational transitions of the dielectric medium and the dielectric absorbs the energy to perform such transition and the energy dissipates due to frictional interactions with the matrix. In principle, the energy loss in electric transmission lines has two components, one is related to the conduction properties of the transmission line, and the other one is related to the dielectric properties of it and is frequency dependent.
| (9) |
| (10) |
where is the dielectric loss factor, is the frequency, D is the inner diameter, and is the outer diameter.
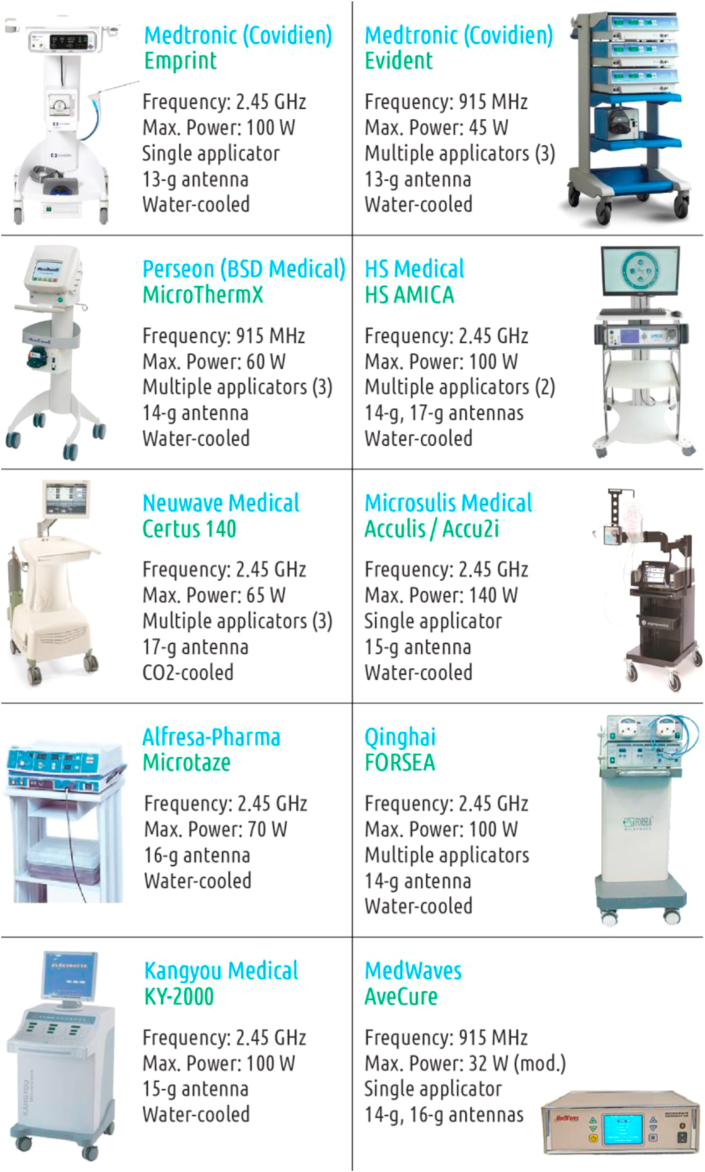
2.6. Present commercial devices
There are currently nine widely used commercial microwave ablation systems used for hepatic cancer. In the United States, the most used FDA-approved system is Covidien product [76]. Figure 6 lists the common commercial MWA systems with their specs. The 915 MHz Covidien MWA system by Vivant Medical, Inc. (Valleylab) was approved by FDA (Food and Drug Administration) in 2003 and later, it was acquired by Covidien plc which was purchased by Medtronic. In the U.S., MWA devices, which are commonly classified as Class II medical devices, require the FDA premarket notification 510(k) clearance as a summary of safety and effectiveness report. A MWA system can deliver microwave radiation to one or more than one applicator. Small tumors commonly are ablated by using a single antenna at a single position. However, in some situations, larger ablation zones are needed for large, multiple, or non-spherical tumors, requiring irradiation at multiple antenna positions [74]. With multiple applicator MWA systems, a single MWA unit connected to two or more antennas can be used simultaneously to achieve a better temperature gradient for a larger zone.
Figure 6.
Common commercial microwave ablation systems.
MedWaves uses a modulated power delivery system. It means that the generator generates maximum power until the temperature sensed by a thermocouple at the distal tip of the antenna reaches 110 °C. Then, the power is modulated to maintain the temperature [77].
3. Percutaneous microwave ablation of liver tumors
The percutaneous approach provides a minimally invasive procedure compared to laparoscopic and laparotomic approaches. In percutaneous microwave ablation techniques, the antenna is positioned at the tumor site, guided by imaging techniques including ultrasound (US) imaging and computer tomography (CT) scanning. Although ultrasound sonography is used in all types of HCC treatments, in some cases, such as small tumors (≤1 cm) or tumors located below the diaphragm, US is not an option [78]. CT-guided MWA compared to US-guided MWA, provides a better resolution of the anatomy and the antenna position, as well as a better visualization for small ultrasonically invisible HCCs [79]. Safety and effectiveness of microwave ablation has been of concern to physicians. Safety in terms of causing damage to surrounding healthy tissues, especially in ablation areas abutting other vital organs, such as the diaphragm, lung, and heart [80]. In cases where the tumor is adjacent to these vital organs, techniques such as artificial ascites or intraductal saline perfusion may be applied. Post-MWA follow-ups of the patients are typically done a few months to 2 years after the ablation by using CT and MRI (Magnetic Resonance Imaging) scans [36].
A research group surveyed the safety and effectiveness of percutaneous MWA of hepatic tumors abutting to the diaphragm and have showed that it is a safe technique in terms of not causing damage to the diaphragm [81]. Percutaneous microwave ablation was performed under general anesthesia using Certus 140 MWA system by NeuWave Medical. Imaging guidance for antenna placement was achieved by either ultrasound (E9, GE Healthcare) or CT fluoroscopy (Light-Speed Advantage, GE Healthcare). Even when the percutaneous approach is not feasible, it has been shown that laparoscopic approach is still a safe and effective method in MWA treatment of HCC tumors [50]. MWA was performed using AMICA-GEN and a single 14- or 16-gauge transcutaneous, AMICA PROBE water-cooled antenna. In a recent study, 220 patients diagnosed with HCC, underwent CT-guided MWA using Qinghai Ltd. MWA system [47]. There were 390 tumor nodules in total with the dimension range of 9–62 mm with an average of 30 mm (188 nodules <30 mm, 166 nodules 30–50 mm, and 36 nodules ≥50 mm). Table 2 shows the effectiveness of MWA from this study. The Qinghai MWA system in this study. The antenna shaft was kept cool by circulating 4 °C saline solution driven by a Qinghai with the rate of 40 ml/min through dual channels inside the antenna shaft.
Table 2.
Complete ablation success rate in a recent study [47]. Complete ablation is defined as no enhancement in the ablative area as at abdominal triphasic CE-CT/CE-MRI 1 month after ablation.
| Tumors' condition | Complete ablation success rate |
|---|---|
| Tumors with a diameter <50 mm | 100% |
| Tumors with a diameter ≥50 mm | 79% |
| Tumors close to key anatomical sites or organs (lungs, porta hepatis, diaphragm, gallbladder, kidney, intestine, and stomach) | 89% |
All patients were followed up for 2 years. Table 3 shows the recurrence rates observed in patients within two years after MWA.
Table 3.
The recurrence rate in patients after a 2-year follow-up in a recent study [47].
| Patients' condition | Recurrence rate |
|---|---|
| All patients | 28% |
| Patients with a tumor diameter ≤30 mm | 13% |
| Patients with a tumor diameter 30–50 mm | 30% |
| Patients with a tumor diameter >50 mm | 60% |
As it is shown in the results, large tumors (tumor diameter >50 mm) have less chance of success and more chance of recurrence compared to smaller ones. In another study which was focused on MWA of large HCC tumors (5–6 cm) in 82 patients, local efficacy and long-term outcomes were investigated [48]. A Qinghai FORSEA MWA system with a 14-gauge cooled shaft antenna was used in this study. It was shown that MWA of large tumors is still a safe procedure with a good efficacy rate of 89% after ablation. On the other hand, long-term outcomes of patients with large tumors are still a matter of concern, as the follow-ups showed that one, three, and five years after ablation, the OS rates were about 93%, 63%, and 41% respectively. We believe the reason for the higher than average 1-year OS rate for such large tumors is the use of a cooled shaft antenna. A cooled shaft antenna provides a better and larger ablation zone by reducing the risk of surface tissue burning and giving a better control over the ablation zone during treatment [82].
The results of CT-guided MWA of 48 patients with tumors in the hepatic dome were evaluated in a recent study [80]. 42 of all patients were diagnosed with HCC. Four different 2.45 GHz MWA systems were used in this study: AMICA by HS Medical, NeuWave by NeuWave Medical, Acculis Microwave Tissue Ablation (MTA) system by AngioDynamics, and Covidien. Microwave antennae ranged in size from 14- to 16-g. 32 of all patients were treated using AMICA MWA system. The range of tumors size was 9–52 mm with an average of 22 mm. They resulted in a high rate of overall ablation success (94%) with minimum collateral damages to the surrounding tissues after gadolinium-enhanced MRI assessments.
In terms of accuracy and procedural safety of stereotactic CT-guided MWA of liver tumors, 20 patients were surveyed in a study [83]. One of the main concerns are bleeding due to multiple repositioning of the microwave antenna when it is not positioned properly and moreover, respiration may cause some movements in the liver and makes it harder to position the antenna. These respiratory movements were minimized in the patients by using high-frequency jet ventilation (HFJV) technique in which a ventilator delivers very small volumes of tidal short-pulsed gas [84, 85, 86]. They confirmed that the combination of stereotactic CT-guided percutaneous MWA and HFJV as a safe and efficient procedure. A European Conformity-marked navigation system dedicated to stereotactic image-guided procedures was used. The MWA system that was used in this study was Accu2i by Microsulis Medical with a water-cooled antenna.
Different liver conditions (cirrhosis or previous chemotherapy treatment) does not make any significant difference in MWA procedure in terms of safety and effectiveness as was shown in a study on 60 patients with different approaches (percutaneous, laparoscopic, and laparotomic) [87]. A Covidien Emprint MWA system with Thermosphere technology and a 13.5-g antenna was used in this study. They have also assessed MWA in vivo compared to ex vivo models and confirmed the reproducibility of ex vivo models on human based on ablation zone volume and ablation time.
As previously mentioned, US guiding could also be used for MWA. In a study on 30 patients with colorectal liver metastases (CRLM), 43 tumors within the range of 14–100 mm (with mean size of 44 mm) were ablated by using percutaneous US-guided MWA combined with synchronous transcatheter arterial chemoembolization (TACE) [88]. The US-guided MWA performed in this study was by using an ECO-100C water-cooled MWA system by ECO Medical Equipment Co. The complete ablation rate was about 81% and US-guided MWA combined with TACE reported as a safe and effective procedure. However, after a 2-year follow-up, the survival rate was about 24%. In fact, some other recent studies have shown that when TACE is combined with MWA in HCC patients, treatment could have higher response and effectiveness compared to TACE alone, especially in case of large tumors where TACE fails to achieve a complete tumor necrosis [44, 89, 90]. For medium or large tumors, combining TACE with MWA tends to be more effective compared to when TACE is combined with RFA due to larger ablation zone of MWA [44, 89].
In a rather large scale and recent study, the outcomes of US-guided percutaneous MWA in 433 patients diagnosed with HCC and different tumor sizes (≤100 mm) were assessed [49]. The real-time US-guided percutaneous MWA procedures were performed using a 2.45 GHz MTC-3C microwave generator and a 15-g cooled-shaft antenna by Vision Medical. Table 4 shows the complete ablation (CA) results based on the tumor size.
Table 4.
Complete ablation (CA) rates of 427 HCC tumors based on tumor size [49].
| Tumor size | <3 cm | 3–5 cm | >5 cm | Total |
|---|---|---|---|---|
| CA rate | 243 (100%) | 118 (100%) | 66 (91.7%) | 427 (98.6%) |
The follow-ups were done 1, 2, and 3 years after the ablation. After the final follow-up, 72.3% of total patients developed tumor recurrences. In our view, multiple tumor numbers, largeness of tumors (typically >5 cm), and TACE before ablation are the factors that could decrease the OS rate of the patients significantly and therefore these parameters should be investigated in depth in larger and longer studies.
Table 5 summarizes some of the recent reports on MWA treatment of liver tumors. CA rate represents the complete ablation successfulness, and OS represents the overall survival rate after follow-ups.
Table 5.
Summarization of some recent reports on MWA treatment of liver tumors. CA rate represents the complete ablation successfulness, and OS represents the overall survival (OS) rate after follow-ups.
| Report | # patients (# tumors) | Age±SD | Tumor size±SD | Diagnosis | Approach | Guide | CA rate | 1-yr OS |
|---|---|---|---|---|---|---|---|---|
| Z. Wu et al. (2016) | 30 (43) | 61.6 ± 10.3 | 1.4–10.0, 4.4 ± 2.6 |
CRLM | Perc. | US | 81.4% | 46.7% |
| T. Yin et al. (2016) | 220 (390) | 59.2 ± 9.1 | 0.9–6.2, 3.0 ± 2.0 |
HCC | Perc. | CT | 92.8% | 95.4% |
| Y. Xu et al. (2016) | 82 (82) | 58.5 ± 11.7 | 5.1–6.0, 5.6 ± 0.3 |
HCC | Perc. | US | 89% | 92.7% |
| N. Asvadi et al. (2016) | 46 (48) | 64.4 | 0.9–5.2, 2.2 |
HCC (88%)/CRC (12%), hepatic dome | Perc. | CT | 94% | (2-year) 73.9% |
| A. Smolock et al. (2015) | 42 (55) | 59.4 | 1.1–3.9, 2.5 ± 1.4 |
HCC (54%)/Met (42%)/Hepatocellular adenomas (4%) | Perc. | US and CT | 94% | OS time: 11 months |
| J. Engstrand et al. (2017) | 17 (25) | 69.6 ±9.2 |
1.5 ± 0.6 | HCC (65%)/CRC (29%)/GIST (6%) | Perc. | CT | 100% | |
| S. Ma et al. (2017) | 433 | <3 57%, 3-5 28%, >5 15% |
HCC | Perc. | US | 98.6% | 83.5% | |
| S. Gruttadauria et al. (2016) | 35 | 64 ±7.9 |
2.8 ± 1.2 | HCC | Lapa. | US | 75% | 85.7% |
With the results presented in Table 5 as well as above discussions, we can infer a selection criteria, describing which patients would benefit most from MWA. Primarily, tumor size and location must be considered. As seen throughout these studies and mentioned previously, tumors < 3cm appear to have the best rates for overall survival. Recommended tumor classification for MWA treatment can be given by ECOG level (0–2), Barcelona Clinic Liver Cancer (BCLA 0 to A), and/or Child-Pugh score (A to B) [47, 48, 49, 88]. Depending on tumor location (within the tumor or on the surface), MWA would be preferable, but not limited, to tumors buried within the liver [91]. Hepatic resection (HR) would be the preferable method for tumors located on the liver surface, however, poor liver function or cirrhosis often complicates HR procedures, making MWA the optimal choice in such scenarios [92, 93]. Therefore, liver function considerations would be another measure. Studies often check for serum alanine transaminases (<60 U/L), serum aspartate transaminases (<60 U/L), total bilirubin (<25 μmol/L), coagulation malfunction (<5 × 109 platelets/L), prothrombin activity (>50%), and other liver indicators [47, 48, 49, 88]. Such liver function tests may be performed before MWA, 3–7 days after, and 1 month after the procedure. ECOG/BCLA/Child-Pugh classification should also be appropriate indicators of liver condition post MWA [94]. Risks/complications covered in section 4 should also be considered if the patient experiences associated symptomology or could be at risk due to an already poor liver function baseline.
4. Complications of hepatic microwave ablation
As described in sections 1–3 and also by previous research, Microwave ablation is considered as a safe technique with minimum complications compared to surgical resection [95, 96]. Complications associated with the surgical resection are commonly due to the post-surgery poor liver function that is caused by the disrupted vital serum protein production system of the liver. Another major resection complication is bile leakage (BL), which may require interventional radiology [95]. The complication rate of hepatic resection is significant compared to MWA or RFA [95, 97]. On the other hand, radiation therapy techniques that are used for primary or metastatic liver tumors, have their own complications which are commonly due to the fact that a portion of normal liver is also irradiated with high energy particles [98]. This may lead to radiation-induced liver disease (RILD), which usually occurs a few months post radiation therapy [95]. These limitations of conventional radio therapy have led to developing new techniques to minimize these collateral damages by trying to localize the radiation inside the tumor such as selective internal radiation therapy (SIRT) [98].
On the contrary, the localized nature of MWA could make it a safer option compared to the radiation therapy techniques. MWA complications resemble those of RFA due to similarity of the two techniques and using thermal energy to destroy malignant cells [98]. Major complications are those that significantly impact scale and duration of post-ablation care, hospitalization and patient morbidity. Major complications of MWA may be categorized into four groups: vascular, biliary, mechanical and infectious [99]. Vascular and biliary are those that are result of damage to blood vessels or bile ducts respectively due to physical damage from antenna or microwave thermal damage. Mechanical complications include collateral damages to surrounding tissues such as gallbladder, diaphragm, colon and stomach. Infectious and functional complications are related to infection of surrounding tissue and ablation zone, respectively.
Death as a result of MWA is extremely rare. However, in a very recent report in 2019, following MWA of HCC in a liver transplant patient with bilioenteric anastomosis (BEA), death was caused by septic shock [100]. In a study of complications of percutaneous MWA on 554 patients with various tumor size and types, no deaths were reported, but major complications were reported in 17 (3%) cases [101]. In the study of 433 HCC patients with different tumor sizes (≤100 mm) treated by percutaneous MWA which was mentioned previously, 23 (5.3%) encountered major complications such as renal insufficiency, hyperbilirubinemia, and reactive pleural effusion [49]. The complication rate was significantly higher in patients with large HCC tumors (>5 cm) compared to patients with smaller tumors. The authors proposed that the reason might be hyperbilirubinemia and renal failure due to releasing large amounts of bilirubin and other cellular components in ablating large tumors.
Many MWA complications arise from lacking sufficient control over the microwave radiation zone. Even when the microwave antenna is accurately in place by using ultrasound or CT imaging, during the MWA procedure, a portion of surrounding healthy tissue can be also affected by the microwave radiation. This collateral damage is more crucial when the ablation zone is adjacent to a vital organ. However, there are promising novel designs with targeting these limitations for MWA that offer minimized collateral damage, among which, are new methods offering temperature-controlled microwave radiation therapy [13]. Currently, the major complications of hepatic MWA are rather scarce with averaging around 5% of all cases reviewed by the author. Using microwave radiation still remains as a safe and effective technique for ablating liver tumors as well as other solid tumors and by using novel designs and minimizing the major complications, it can become an unrivaled technique in this scope.
5. Microwave ablation and COVID-19
Since the emergence of the COVID-19 pandemic, one of the concerning risk factors has been COVID-19-associated liver injury which can be a result of a pre-existing liver condition and/or a worsening liver condition during COVID-19 illness and treatment [102]. The worsening liver condition could be attributed to the following effects:
- 1)
-
2)
Possible cytotoxic activities of SARS-CoV-2 viral materials in the liver [105, 106].
-
3)
Drug-induced liver injury (DILI) associated with anti-COVID-19 drugs such as remdesivir, tocilizumab, lopinavir, chloroquine and hydroxychloroquine [105, 107, 108].
The effects of the SARS-CoV-2 virus on the host's immune system has been a significant area of research in order to understand the disease's progression and treatments. The S-glycoprotein of this virus consists of two subunits, S1 and S2, each of which are shown to partake in the binding process to ACE2 receptors on the cell membrane [109]. Heptad repeat 1 (HR1) and heptad repeat 2 (HR2) interact with the S2 subunit, organizing into a six-helix bundle (6HB), guiding the S1 receptor binding domain onto the ACE2 receptor [110, 111]. From here, the S1 and S2 units are cleaved by the intracellular protease TMPRSS2, followed by endocytosis of the virus into the cell [109]. Upon gaining entry, viral RNA is released, and the replication process of SARS-CoV-2 begins. Viral release from the infected cells results in apoptosis, subsequently triggering the release of a “cytokine storm”, involving the elevation of IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A and TNF-α [112]. Additionally, this elevated cytokine situation induces inflammatory CD14 + CD16 + monocytes promoting a positive feedback expression of IL-6, further accelerating inflammation and organ damage [113].
However, there are conflicting opinions between literature describing whether or not this particular virus is capable of infecting hepatocytes, directly causing liver dysfunction [114, 115, 116, 117]. The paper published by Wang and colleagues presents data indicating the presence of viral materials within such hepatocytic structures, but the relatively low ACE2 gene expression within the liver, and seemingly analogous structures between cell and viral materials has been a cause for debate on these results [114, 115, 116, 117]. With that said, it's reasonable to suggest that the SARS-CoV-2 virus is capable of hijacking ACE2 receptors in the liver, even with low expression. For example, despite differences between low lung and high intestine ACE2 gene expression, it's observed that severe respiratory symptomology is unequivocally dominant during sustained infections. This suggests additional factors are associated with viral load, other than ACE2 expression, which may also indicate a similar mechanism is occurring in the liver [118, 119, 120].
Considerations to make when evaluating the susceptibility and severity of patient and organ infection would be potential up/down regulation of ACE2 gene expression upon viral entry into subjects with or without cancer. While there doesn't appear to be information on the expression level of ACE2 specifically within virally infected liver cancer cells, it has been determined that the engagement of the SARS-CoV spike protein (the 2002 variant) downregulates ACE2 expression on cell membranes, and a similar process is at play with the current virus [121, 122, 123]. This down-regulation results in an imbalance between the RAS and ACE2/Ang-II/Ang-(1–7)/MAS pathway, contributing to dysregulated inflammation and ultimately, organ damage [122, 124]. Additionally, it has also been shown that ACE2 is downregulated in a variety of tumour types, including HCC, which is associated with poor prognosis for this cancer type [125, 126]. Interestingly, administration of human recombinant soluble ACE2 (hrsACE2) has shown promising results in treatment of the virus, likely due to hrsACE2 intercepting the virus before it can interact with membrane bound ACE2 [121,127]. Seeing as a downregulation of ACE2 correlates with a poor outcome for COIVD-19 and HCC patients, perhaps it would be of interest to develop a method involving supra-physiological/exogenous injections of hrsACE2, combined with MWA to the malignant area, for those suffering from both the virus and HCC.
One of the largest cohort studies enrolling 1,099 COVID-19 patients in China suggests statistical significance of some characteristics and variables associated with liver conditions. The study showed that 21 (2.1%) had hepatitis B infection. Moreover, 22.2% (168/757) and 21.3% (158/741) showed concentration elevation of transaminase enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), respectively. These elevations were more significant in severe patients compared to non-severe patients for both AST (39.4% vs 18.2%) and ALT (28.1% vs 19.8%). Elevated bilirubin levels were also found in 10.5% (76/722) of the patients [128]. Highest levels of AST can be found in liver and skeletal muscle. On the other hand, ALT is predominantly present in liver, followed by kidney and skeletal muscle [129]. Therefore, one of the main reasons for AST and ALT elevations in serum are associated with liver injury and secretion of the enzymes into extracellular space [130]. Serum bilirubin is another biomarker where high levels could be an indication of liver diseases [131]. All these biomarkers have been shown to be related to HCC, as the most prevalent type of liver cancer [132]. Other studies have shown considerable rates of liver dysfunction in COVID-19 patients which resulted in longer hospitalization times [133, 134]. However, the extent of HCC, and in general, liver injury as a COVID-19 risk factor is still unclear [105, 135]; for example, susceptibility to COVID-19 for patients with pre-existing liver disease due to compromised immune system [134], severity of COVID-19 in these patients, and the role of the virus in liver injury and deteriorating liver conditions are not completely understood [105]. Nevertheless, one major concern during pandemics like COVID-19 would be the negative impact on quality of care and mortality rates for COVID-19 patients with underlying liver conditions [136].
To combat these uncertainties and concerns, MWA in COVID-19 patients admitted to hospitals suffering with early stage hepatic malignancies may be an optimal solution. Such a treatment during these times is actually recommended in HCC classified by the BCLC as BCLC 0 or A, where these subcategories include tumours <3 cm [137]. However, perhaps larger or multiple growths can be efficiently ablated by using more effective antenna designs or simultaneous application of multiple antennas [138]. Further advantages of MWA can be attributed to its shorter postoperative stay in the ICU and lower risk of complications, compared to surgical resection or liver transplantation, leaving more clinical resources available during pandemics [9, 137, 139].
The use of combination therapies with MWA, such as ablation induced immunomodulation/immunotherapy, would be another consideration for treating HCC patients suffering from COVID-19. Unfortunately, there appears to be no published studies linking the effectiveness of these treatment combinations in HCC subjects with the virus. Nonetheless, effects of MWA and immunotherapies have been explored independently of COVID-19, so assumptions can be made until further data is available.
Thermal ablation of tumours generates an antitumor response through a process known as the abscopal effect, which is capable of eliminating remaining malignant cells post ablation to reduce tumour reoccurrences [140]. This effect is quite weak on its own and can be combined with immunotherapies to significantly strengthen it, but a possible concern lies within over activating the immune response in those diagnosed with COVID-19. Patients with severe symptoms of the virus can experience cytokine storm syndrome (CSS), resulting in upregulation of inflammatory proteins (i.e. IL-6 and many others), ultimately leading to organ failure and death if not controlled [141, 142]. However, part of the usefulness of MWA in the treatment of HCC is the ability for this method to induce the abscopal effect [143, 144]. Following certain ablation techniques, such as RFA or MWA, secretion of IL-1, IL-6, and HSP 70 are all elevated, but the effect in MWA is significantly less compared to its other ablation counterparts [144]. This is a positive outcome in terms of treating cancer patients with COVID-19, as a weaker immune response would be safer for patients with severe symptoms. Therefore, MWA is probably preferred to RFA for these patients. Conversely, this may imply that MWA is less effective at reducing tumour reoccurrences compared to RFA. Yet, this is not the case, likely because the abscopal effect is already rather weak on its own, as stated earlier. Additionally, two meta-analysis studies in 2019 comparing MWA and RFA concluded that MWA has lower tumour reoccurrence, which may be due to the nature of MWA, as it is able to produce a more uniform ablation pattern and larger tumour necrosis. The shape and size of the ablation zone in RFA is often unpredictable and can lead to an insufficient ablation zone area in comparison [145, 146].
Using immunotherapy compounds or immune checkpoint inhibitors (ICIs) to treat cancer in patients with COVID-19 is also a topic that is still being repeatedly explored [147, 148, 149, 150]. Based on current literature summarized by Gambichler and colleagues, they essentially concluded that the administration of ICIs such as PD-1 inhibitors and anti-CTLA-4 compounds show no risk of increased vulnerability of COVID-19 infection [147]. There does appear to be some support that the use of PD-1 inhibitors on CD8+ lymphocytes could actually be beneficial to those suffering from both cancer and early stages of COVID-19, as this ICI treatment can restore cytotoxicity within tumours and virally infected cells through decreasing T cell exhaustion [148]. Although, the data is limited and conflicting when it comes to treating patients with ICIs if they have both the virus and cancer, it seems that combination of immunotherapy compounds and MWA could be a suitable option for those who do not have severe Covid-19 infections.
6. Conclusions
This review aimed at the recent reports on MWA treatment of liver tumors. All of them confirm the safety and effectiveness of this technique. The preferable approach is percutaneous, but even when percutaneous approach is not feasible; the laparoscopic approach is still safe and effective. Commonly for small tumors (<3 cm), a single antenna insertion is sufficient whereas for larger tumors, multiple overlapped ablations may be required. The complete ablation (CA) rates for small tumors (<3 cm) are relatively very high with the minimum complication rate. However, for larger tumors, especially tumors larger than 5 cm in diameter, the completion rates and the OS rates decrease, while the complication rates and recurrence rates increase. Regardless of number and type of the antennas, 915 MHz is recommended for large tumors whereas for small tumors, 2.45 GHz provides more localized ablation zone, sharper ablation zone and with less damage to the surrounding tissue. 2.45 GHz is also recommended for tumors close to the vital organs. Based on section 3 and the complications mentioned in section 4, MWA is recommended over other techniques including surgical resection for tumors up to about 5 cm and when the tumor is not adjacent to vital organs.
One problem with the percutaneous microwave ablation is forming undesired skin burns around the inserted antenna and one effective way to reduce this problem is to use double-slot antenna with metallic choke. The metallic choke reflects the unwanted radiation from the tip of the antenna towards the surface of the body and the skin. Another effective way to minimize the risk of surface tissue burns, is to use a cooled shaft antenna. A cooled shaft antenna has also shown to be more effective for large tumors by providing a better and larger ablation zone.
For COVID-19 patients admitted to hospitals, it is recommended to perform liver function tests including ALT and AST, and bilirubin measurements to evaluate the risk factors and consider MWA. More rigorous monitoring and assessments are suggested for cases where the patient is treated with drugs including remdesivir, tocilizumab, lopinavir, chloroquine and hydroxychloroquine.
Considerations and compliance with ethical standards
-
o
This article is not under consideration for publication elsewhere.
-
o
This research did receive grants from NSERC, University of Guelph COVID-19 Research Development and Catalyst Fund and MITACS.
-
o
This article does not contain any studies with human participants or animals performed by any of the authors.
-
o
For this type of study consent for publication is not required.
-
o
Publication is approved by all authors.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by NSERC, University of Guelph COVID-19 Research Development and Catalyst Fund, and MITACS.
Data availability statement
Data will be made available upon request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. Farkhondeh Fathi for the initial discussions related to some of the topics in this review paper. Pooya Afaghi started this review under supervision of Dr. Ghandi in a class that was offered by Dr. Fathi.
References
- 1.Liang P., Yu X., Yu J., editors. Microwave Ablation Treatment of Solid Tumors. 2015. [Google Scholar]
- 2.Chu K.F., Dupuy D.E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 3.Keefe N.A., Haskal Z.J., Park A.W., Angle J.F., editors. IR Playbook: A Comprehensive Introduction to Interventional Radiology. 2018. [Google Scholar]
- 4.Liu C., Wang Y., Yu X., Dong B., Zhou P., Ren H., Liang P. Is percutaneous microwave ablation of liver tumor safe for patients with renal dysfunction. Eur. J. Radiol. 2011 doi: 10.1016/j.ejrad.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Chen M., Zhang Y., Lau W.Y., editors. Radiofrequency Ablation for Small Hepatocellular Carcinoma. 2016. [Google Scholar]
- 6.Ding J., Jing X., Liu J., Wang Y., Wang F., Wang Y., Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin. Radiol. 2013;68:608–615. doi: 10.1016/j.crad.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Kang T.W., Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015;4:176–187. doi: 10.1159/000367740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Gregorio M.A., Guirola J.A., Magallanes M. COVID-19 outbreak: infection control and management protocol for vascular and interventional radiology departments—consensus document. Cardiovasc. Intervent. Radiol. 2020 doi: 10.1007/s00270-020-02493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iavarone M., Sangiovanni A., Carrafiello G., Rossi G., Lampertico P. Management of hepatocellular carcinoma in the time of COVID-19. Ann. Oncol. 2020;1–2 doi: 10.1016/j.annonc.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santambrogio R., Farina G., D’Alessandro V., Iacob G., Gemma M., Zappa M.A. Guidelines adaptation to the COVID-19 outbreak for the management of hepatocellular carcinoma. J. Laparoendosc. Adv. Surg. Tech. lap. 2020;2020:559. doi: 10.1089/lap.2020.0559. [DOI] [PubMed] [Google Scholar]
- 11.Li M., Zhu N. Recent advances in microwave photonics. Front. Optoelectron. 2016;9:160–185. [Google Scholar]
- 12.Rosen A., Stuchly M.A., Vander Vorst A. Applications of RF/microwaves in medicine. IEEE Trans. Microw. Theor. Tech. 2002;50:963–974. [Google Scholar]
- 13.Ghandi K., Afaghi P. 2018. Systems and Methods for Use and Measurement of Non-thermal Effects of Microwave Radiation. US Patent 20200068672, Filed. [Google Scholar]
- 14.Chandrasekaran S., Ramanathan S., Basak T. Microwave food processing—a review. Food Res. Int. 2013;52:243–261. [Google Scholar]
- 15.Kim J., Mun S.C., Ko H.U., Kim K.B., Khondoker M.A.H., Zhai L. Review of microwave assisted manufacturing technologies. Int. J. Precis. Eng. Manuf. 2012;13:2263–2272. [Google Scholar]
- 16.Vander Vorst A., Rosen A., Kotsuka Y. RF/Microwave interaction with biological tissues. RF/Microwave Interact. Biol. Tiss. 2006 [Google Scholar]
- 17.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 19.Stewart B.W., Wild C.P., editors. World Cancer Report 2014. International Agency for Research on Cancer; Lyon: 2014. [Google Scholar]
- 20.Schulze K., Nault J.C., Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J. Hepatol. 2016;65:1031–1042. doi: 10.1016/j.jhep.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Custer B., Sullivan S.D., Hazlet T.K., Iloeje U., Veenstra D.L., Kowdley K.V. Global epidemiology of hepatitis B virus. J. Clin. Gastroenterol. 2004;38:S158–S168. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- 22.Shepard C.W., Finelli L., Alter M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 23.Te H.S., Jensen D.M. Epidemiology of hepatitis B and C viruses: a global overview. Clin. Liver Dis. 2010;14:1–21. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Cornberg M., Razavi H.A., Alberti A. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31:30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 25.Shafizadeh N., Kakar S. Diagnosis of well-differentiated hepatocellular lesions: role of immunohistochemistry and other ancillary techniques. Adv. Anat. Pathol. 2011;18:438–445. doi: 10.1097/PAP.0b013e318234abb4. [DOI] [PubMed] [Google Scholar]
- 26.Schlageter M., Terracciano L.M., D’Angelo S., Sorrentino P. Histopathology of hepatocellular carcinoma. World J. Gastroenterol. 2014;20:15955–15964. doi: 10.3748/wjg.v20.i43.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandrine V., Benoit T., Laura R.-B. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017:22–34. doi: 10.21037/hbsn.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong B.W., Liang P., Yu X.L., Zeng X.Q., Wang P.J., Su L., Wang X.D., Xin H., Li S. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. Am. J. Roentgenol. 1998;171:449–454. doi: 10.2214/ajr.171.2.9694473. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiki N. Histopathological changes after microwave coagulation therapy for patients with hepatocellular carcinoma: review of 15 explanted livers. Am. J. Gastroenterol. 2003;98:2052–2059. doi: 10.1111/j.1572-0241.2003.07642.x. [DOI] [PubMed] [Google Scholar]
- 30.Ton J., Kuoy E., Abi-jaoudeh N. IR playbook: liver ablation. IR Playb. 2018:397–403. [Google Scholar]
- 31.Amabile C., Farina L., Lopresto V., Pinto R., Cassarino S., Tosoratti N., Goldberg S.N., Cavagnaro M. Tissue shrinkage in microwave ablation of liver: an ex vivo predictive model. Int. J. Hyperther. 2017;33:101–109. doi: 10.1080/02656736.2016.1208292. [DOI] [PubMed] [Google Scholar]
- 32.Chinn S.B., Lee F.T., Kennedy G.D., Chinn C., Johnson C.D., Winter T.C., Warner T.F., Mahvi D.M. Effect of vascular occlusion on radiofrequency ablation of the liver. Am. J. Roentgenol. 2001;176:789–795. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- 33.Poulou L.S., Botsa E., Thanou I., Ziakas P.D., Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J., Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 35.Shi J., Sun Q., Wang Y., Jing X., Ding J., Yuan Q., Ren C., Shan S., Wang Y., Du Z. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J. Gastroenterol. Hepatol. 2014;29:1500–1507. doi: 10.1111/jgh.12572. [DOI] [PubMed] [Google Scholar]
- 36.Poggi G., Tosoratti N., Montagna B., Picchi C. Microwave ablation of hepatocellular carcinoma. World J. Hepatol. 2015;7:2578–2589. doi: 10.4254/wjh.v7.i25.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crocetti L. Thermal ablation of hepatocellular carcinoma. Canc. Imag. 2008;8:19–26. doi: 10.1102/1470-7330.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright A.S., Lee F.T., Mahvi D.M. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann. Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Wright A.S., Sampson L.A., Warner T.F., Mahvi D.M., Lee F.T. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 40.Martin R.C.G., Scoggins C.R., McMasters K.M. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann. Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 41.Andreano A., Brace C.L. A comparison of direct heating during radiofrequency and microwave ablation in Ex vivo liver. Cardiovasc. Intervent. Radiol. 2013;36:505–511. doi: 10.1007/s00270-012-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornelis F.H., Durack J.C., Kimm S.Y., Wimmer T., Coleman J.A., Solomon S.B., Srimathveeravalli G. A comparative study of ablation boundary sharpness after percutaneous radiofrequency, cryo-, microwave, and irreversible electroporation ablation in normal swine liver and kidneys. Cardiovasc. Intervent. Radiol. 2017;40:1600–1608. doi: 10.1007/s00270-017-1692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W., Zheng Y., He W. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment. Pharmacol. Ther. 2018;48:671–681. doi: 10.1111/apt.14929. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z., Xie H., Zhou L., Chen X., Zheng S. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal. Cell Pathol. 2019;2019:1–7. doi: 10.1155/2019/8619096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keane F.K., Hong T.S. Role and future directions of external beam radiotherapy for primary liver cancer. Cancer Control. 2017;24 doi: 10.1177/1073274817729242. 107327481772924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukumitsu N., Okumura T., Takizawa D. Proton beam therapy for metastatic liver tumors. Radiother. Oncol. 2015;117:322–327. doi: 10.1016/j.radonc.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Yin T., Li W., Zhao P., Wang Y., Zheng J. Treatment efficacy of CT-guided percutaneous microwave ablation for primary hepatocellular carcinoma. Clin. Radiol. 2017;72:136–140. doi: 10.1016/j.crad.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y., Shen Q., Wang N., Liu P., Wu P., Peng Z., Qian G. Percutaneous microwave ablation of 5-6 cm unresectable hepatocellular carcinoma: local efficacy and long-term outcomes. Int. J. Hyperther. 2016;33:247–254. doi: 10.1080/02656736.2016.1239842. [DOI] [PubMed] [Google Scholar]
- 49.Ma S., Ding M., Li J. Ultrasound - guided percutaneous microwave ablation for hepatocellular carcinoma : clinical outcomes and prognostic factors. J. Canc. Res. Clin. Oncol. 2017;143:131–142. doi: 10.1007/s00432-016-2266-5. [DOI] [PubMed] [Google Scholar]
- 50.Gruttadauria S., Pagano D., Tropea A., Cintorino D., Castellana L., Bonsignore P., Ricotta C., Piccolo G., Vizzini G., Luca A. Laparoscopic approach for thermoablation microwave in the treatment of hepatocellular carcinoma: a single center experience. J. Laparoendosc. Adv. Surg. Tech. 2016;26 doi: 10.1089/lap.2016.0373. lap.2016.0373. [DOI] [PubMed] [Google Scholar]
- 51.Chiba T., Tokuuye K., Matsuzaki Y. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin. Canc. Res. 2005;11:3799–3805. doi: 10.1158/1078-0432.CCR-04-1350. [DOI] [PubMed] [Google Scholar]
- 52.Purdom L., Ambrose E.J., Klein G. A correlation between electrical surface charge and some biological characteristics during the stepwise progression of a mouse sarcoma. Nature. 1958;181:1586–1587. doi: 10.1038/1811586a0. [DOI] [PubMed] [Google Scholar]
- 53.Joines W.T., Jirtle R.L., Rafal M.D., Schaefer D.J. Microwave power absorption differences between normal and malignant tissue. Int. J. Radiat. Oncol. Biol. Phys. 1980;6:681–687. doi: 10.1016/0360-3016(80)90223-0. [DOI] [PubMed] [Google Scholar]
- 54.O ’rourke A.P., Lazebnik M., Bertram J.M., Converse M.C., Hagness S.C., Webster J.G., Mahvi D.M. Dielectric properties of human normal, malignant and cirrhotic liver tissue: in vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007;52:4707–4719. doi: 10.1088/0031-9155/52/15/022. [DOI] [PubMed] [Google Scholar]
- 55.Keangin P., Rattanadecho P., Wessapan T. An analysis of heat transfer in liver tissue during microwave ablation using single and double slot antenna. Int. Commun. Heat Mass Tran. 2011;38:757–766. [Google Scholar]
- 56.Liang P., Yu J., Lu M De. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J. Gastroenterol. 2013;19:5430–5438. doi: 10.3748/wjg.v19.i33.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertram J.M., Yang D., Converse M.C., Webster J.G., Mahvi D.M. A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit. Rev. Biomed. Eng. 2006;34:187–213. doi: 10.1615/critrevbiomedeng.v34.i3.10. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y., Cheng Z., Dong L., Zhang G., Wang Y., Liang P. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine livers. Eur. J. Radiol. 2012;81:553–557. doi: 10.1016/j.ejrad.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Simo K.A., Tsirline V.B., Sindram D., McMillan M.T., Thompson K.J., Swan R.Z., McKillop I.H., Martinie J.B., Iannitti D.A. Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB. 2013;15:991–996. doi: 10.1111/hpb.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curto S., Taj-Eldin M., Fairchild D., Prakash P. Microwave ablation at 915 MHz vs 2.45 GHz: a theoretical and experimental investigation. Med. Phys. 2015;42:6152. doi: 10.1118/1.4931959. [DOI] [PubMed] [Google Scholar]
- 61.Balanis C.A. fourth ed. Wiley; 2016. Antenna Theory: Analysis and Design. [Google Scholar]
- 62.Jones R.P., Kitteringham N.R., Terlizzo M., Hancock C., Dunne D., Fenwick S.W., Poston G.J., Ghaneh P., Malik H.Z. Microwave ablation of ex vivo human liver and colorectal liver metastases with a novel 14.5 GHz generator. Int. J. Hyperther. 2012;28:43–54. doi: 10.3109/02656736.2011.610428. [DOI] [PubMed] [Google Scholar]
- 63.Hancock C.P., Burn P., Duff C.I. A new wave in electrosurgery: a review of existing and introduction to new radio-frequency and microwave therapeutic systems. IEEE Microw. Mag. 2015;16:14–30. [Google Scholar]
- 64.Hancock C.P., Dharmasiri N., White M., Goodman A.M. The design and development of an integrated multi-functional microwave antenna structure for biological applications. IEEE Trans. Microw. Theor. Tech. 2013;61:2230–2241. [Google Scholar]
- 65.Gabriel S., Lau R.W., Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 66.Brace C.L. Microwave tissue ablation: biophysics, technology, and applications. Crit. Rev. Biomed. Eng. 2010;38:65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longo I., Gentili G.B., Cerretelli M., Tosoratti N. A coaxial antenna with miniaturized choke for minimally invasive interstitial heating. IEEE Trans. Biomed. Eng. 2003;50:82–88. doi: 10.1109/TBME.2002.807320. [DOI] [PubMed] [Google Scholar]
- 68.Maini S., Marwaha A. Proc 2011 World Congr Inf Commun Technol WICT 2011. 2011. Comparison of coaxial choke and extended tip choke antenna for interstitial microwave ablation of HCC; pp. 841–845. [Google Scholar]
- 69.Lin J.C., Wang Y.J. The cap-choke catheter antenna for microwave ablation treatment. IEEE Trans. Biomed. Eng. 1996;43:657–660. doi: 10.1109/10.495286. [DOI] [PubMed] [Google Scholar]
- 70.Bertram J.M., Yang D., Converse M.C., Webster J.G., Mahvi D.M. Antenna design for microwave hepatic ablation using an axisymmetric electromagnetic model. Biomed. Eng. Online. 2006;5:15. doi: 10.1186/1475-925X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiu H.M., Mohan A.S., Weily A.R., Guy D.J.R., Ross D.L. Analysis of a novel expanded tip wire (ETW) antenna for microwave ablation of cardiac arrhythmias. IEEE Trans. Biomed. Eng. 2003;50:890–899. doi: 10.1109/TBME.2003.813541. [DOI] [PubMed] [Google Scholar]
- 72.Ibitoye Z.A., Nwoye E.O., Aweda M.A., Oremosu A.A., Annunobi C.C., Akanmu O.N. Optimization of dual slot antenna using floating metallic sleeve for microwave ablation. Med. Eng. Phys. 2015;37:384–391. doi: 10.1016/j.medengphy.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Brace C.L., Laeseke P.F., Van Der Weide D.W., Lee F.T. Microwave ablation with a triaxial antenna: results in ex vivo Bovine Liver. IEEE Trans. Microw. Theor. Tech. 2005;53:215–220. doi: 10.1109/TMTT.2004.839308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brace C.L., Laeseke P.F., Sampson L.A., Frey T.M., van der Weide D.W., Lee F.T. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 75.Brace C.L., van der Weide D.W., Lee F.T., Laeseke P.F., Sampson L. Vol. 144. 2008. Analysis and experimental validation of a triaxial antenna for microwave tumor ablation; pp. 1437–1440. (2004 IEEE MTT-S Int Microw Symp Dig (IEEE Cat No04CH37535)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lubner M.G., Brace C.L., Hinshaw J.L., Lee F.T. Microwave tumor ablation: mechanism of action, clinical results, and devices. J. Vasc. Intervent. Radiol. 2010;21:S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf F.J., Aswad B., Ng T., Dupuy D.E. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology. 2012;262:353–360. doi: 10.1148/radiol.11110015. [DOI] [PubMed] [Google Scholar]
- 78.Sato M., Watanabe Y., Tokui K., Kawachi K., Sugata S., Ikezoe J. CT-guided treatment of ultrasonically invisible hepatocellular carcinoma. Am. J. Gastroenterol. 2000;95:2102–2106. doi: 10.1111/j.1572-0241.2000.02275.x. [DOI] [PubMed] [Google Scholar]
- 79.Kim Y.K., Kim C.S., Lee J.M., Chung G.H., Chon S Bin. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur. J. Radiol. 2006;60:100–107. doi: 10.1016/j.ejrad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Asvadi N.H., Anvari A., Uppot R.N., Thabet A., Zhu A.X., Arellano R.S. CT-guided percutaneous microwave ablation of tumors in the hepatic dome: assessment of efficacy and safety. J. Vasc. Intervent. Radiol. 2016;27:496–502. doi: 10.1016/j.jvir.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Smolock A.R., Lubner M.G., Ziemlewicz T.J., Hinshaw J.L., Kitchin D.R., Brace C.L., Lee F.T. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. Am. J. Roentgenol. 2014;204:197–203. doi: 10.2214/AJR.14.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuang M., Lu M.D., Xie X.Y., Xu H.X., Mo L.Q., Liu G.J., Xu Z.F., Zheng Y.L., Liang J.Y. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna—experimental and clinical studies. Radiology. 2007;242:914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]
- 83.Engstrand J., Toporek G., Harbut P., Jonas E., Nilsson H., Freedman J. Stereotactic CT-guided percutaneous microwave ablation of liver tumors with the use of high-frequency jet ventilation: an accuracy and procedural safety study. Am. J. Roentgenol. 2017;208:193–200. doi: 10.2214/AJR.15.15803. [DOI] [PubMed] [Google Scholar]
- 84.Biro P., Spahn D.R., Pfammatter T. High-frequency jet ventilation for minimizing breathing-related liver motion during percutaneous radiofrequency ablation of multiple hepatic tumours. Br. J. Anaesth. 2009;102:650–653. doi: 10.1093/bja/aep051. [DOI] [PubMed] [Google Scholar]
- 85.Denys A., Lachenal Y., Duran R., Chollet-Rivier M., Bize P. Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc. Intervent. Radiol. 2014;37:140–146. doi: 10.1007/s00270-013-0620-4. [DOI] [PubMed] [Google Scholar]
- 86.Abderhalden S., Biro P., Hechelhammer L., Pfiffner R., Pfammatter T. CT-guided navigation of percutaneous hepatic and renal radiofrequency ablation under high-frequency jet ventilation: feasibility study. J. Vasc. Intervent. Radiol. 2011;22:1275–1278. doi: 10.1016/j.jvir.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 87.De Cobelli F., Paolo Marra B., Francesca Ratti B. Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions. Med. Oncol. 2017 doi: 10.1007/s12032-017-0903-8. [DOI] [PubMed] [Google Scholar]
- 88.Wu Z.-B., Si Z.-M., Qian S., Liu L.-X., Qu X.-D., Zhou B., Zhang W., Wang G.-Z., Liu R., Wang J.-H. Percutaneous microwave ablation combined with synchronous transcatheter arterial chemoembolization for the treatment of colorectal liver metastases: results from a follow-up cohort. OncoTargets Ther. 2016;9:3783–3789. doi: 10.2147/OTT.S105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Q.-F., Jia Z.-Y., Yang Z.-Q., Fan W.-L., Shi H.-B. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization–microwave ablation therapy for hepatocellular carcinoma tumors ≤5 cm: a propensity analysis at a single center. Cardiovasc. Intervent. Radiol. 2017;40:1748–1755. doi: 10.1007/s00270-017-1736-8. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R., Shen L., Zhao L., Guan Z., Chen Q., Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn. Interv. Radiol. 2018 doi: 10.5152/dir.2018.17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glassberg M.B., Ghosh S., Clymer J.W., Wright G.W.J., Ferko N., Amaral J.F. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J. Surg. Oncol. 2019 doi: 10.1186/s12957-019-1632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang E.L., Yang F., Wu Z.B., Yue C.S., He T.Y., Li K.Y., Xiao Z.Y., Xiong M., Chen X.P., Huang Z.Y. Therapeutic efficacy of percutaneous microwave coagulation versus liver resection for single hepatocellular carcinoma ≤3 cm with Child-Pugh A cirrhosis. Eur. J. Surg. Oncol. 2016;42:690–697. doi: 10.1016/j.ejso.2016.02.251. [DOI] [PubMed] [Google Scholar]
- 93.Chong C.C.N., Lee K.F., Chu C.M. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: application of ALBI score to patient selection. HPB. 2018;20:546–554. doi: 10.1016/j.hpb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Y., Jing X., Ding J., Wang Y. Risk factors of liver dysfunction induced by microwave ablation in patients with hepatocellular carcinoma. Ultrasound Med. Biol. 2017;43:S162. [Google Scholar]
- 95.Liang P., Wang Y., Yu X., Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 96.Ishii M. Comprehensive review of post-liver resection surgical complications and a new universal classification and grading system. World J. Hepatol. 2014;6:745. doi: 10.4254/wjh.v6.i10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Amerongen M.J., Jenniskens S.F.M., van den Boezem P.B., Fütterer J.J., de Wilt J.H.W. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases – a meta-analysis. HPB. 2017;19:749–756. doi: 10.1016/j.hpb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Maor Y., Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int. J. Hepatol. 2013;2013:1–8. doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang C., Cortis K., Yusuf G.T., Gregory S., Lewis D., Kane P., Peddu P. Complications from percutaneous microwave ablation of liver tumours: a pictorial review. Br. J. Radiol. 2019;92:20180864. doi: 10.1259/bjr.20180864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Melikian R., Minocha J. Septic shock and death after microwave ablation of hepatocellular carcinoma in a liver transplant patient with a bilioenteric anastomosis. Semin. Intervent. Radiol. 2019;36:137–141. doi: 10.1055/s-0039-1688430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Livraghi T., Meloni F., Solbiati L., Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc. Intervent. Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 102.Garrido I., Liberal R., Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment. Pharmacol. Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng G., Zheng K.I., Yan Q.-Q., Rios R.S., Targher G., Byrne C.D., Poucke S Van, Liu W.-Y., Zheng M.-H. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J. Clin. Transl. Hepatol. 2020;8:1–7. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taefehshokr N., Taefehshokr S., Hemmat N., Heit B. Covid-19: perspectives on innate immune evasion. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Q., Zhang Y., Wu L. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Washington I.M., Van Hoosier G. Lab Rabbit Guinea Pig, Hamster, Other Rodents. 2012. Clinical biochemistry and hematology. [Google Scholar]
- 108.Aulbach A.D., Amuzie C.J. A Compr. Guid. to Toxicol. Nonclinical Drug Dev. second ed. Elsevier; 2017. Biomarkers in nonclinical drug development; pp. 447–471. [Google Scholar]
- 109.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S., Xiao G., Chen Y. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia S., Zhu Y., Liu M. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Liu S., Liu H. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bangash M.N., Patel J.M., Parekh D., Murphy N., Brown R.M., Elsharkawy A.M., Mehta G., Armstrong M.J., Neil D. SARS-CoV-2: is the liver merely a bystander to severe disease? J. Hepatol. 2020;73:995–996. doi: 10.1016/j.jhep.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Smet V., Verhulst S., van Grunsven L.A. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS-CoV-2. J. Hepatol. 2020;73:993–995. doi: 10.1016/j.jhep.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Philips C.A., Ahamed R., Augustine P. SARS-CoV-2 related liver impairment – perception may not be the reality. J. Hepatol. 2020;73:991–992. doi: 10.1016/j.jhep.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y., Lu F., Zhao J. Reply to: correspondence relating to “SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020;73:996–998. doi: 10.1016/j.jhep.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 121.Furuhashi M., Moniwa N., Takizawa H., Ura N., Shimamoto K. Potential differential effects of renin-angiotensin system inhibitors on SARS-CoV-2 infection and lung injury in COVID-19. Hypertens. Res. 2020;43:837–840. doi: 10.1038/s41440-020-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Medina-Enríquez M.M., Lopez-León S., Carlos-Escalante J.A., Aponte-Torres Z., Cuapio A., Wegman-Ostrosky T. ACE2: the molecular doorway to SARS-CoV-2. Cell Biosci. 2020;10:148. doi: 10.1186/s13578-020-00519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferreira-Duarte M., Estevinho M.M., Duarte-Araújo M., Magro F., Morato M. Unraveling the role of ACE2, the binding receptor for SARS-CoV-2, in inflammatory bowel disease. Inflamm. Bowel Dis. 2020;26:1787–1795. doi: 10.1093/ibd/izaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye G., Qin Y., Lu X., Xu X., Xu S., Wu C., Wang X., Wang S., Pan D. The association of renin-angiotensin system genes with the progression of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2015;459:18–23. doi: 10.1016/j.bbrc.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 126.Saponaro F., Rutigliano G., Sestito S., Bandini L., Storti B., Bizzarri R., Zucchi R. ACE2 in the era of SARS-CoV-2: controversies and novel perspectives. Front. Mol. Biosci. 2020 doi: 10.3389/fmolb.2020.588618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abd El-Aziz T.M., Al-Sabi A., Stockand J.D. Human recombinant soluble ACE2 (hrsACE2) shows promise for treating severe COVID-19. Signal Transduct. Target Ther. 2020;5:258. doi: 10.1038/s41392-020-00374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gowda S., Desai P.B., Hull V.V., Math A.A.K., Vernekar S.N., Kulkarni S.S. A review on laboratory liver function tests. Pan. Afr. Med. J. 2009;3:17. [PMC free article] [PubMed] [Google Scholar]
- 129.Stepien M., Fedirko V., Duarte-Salles T. Prospective association of liver function biomarkers with development of hepatobiliary cancers. Cancer Epidemiol. 2016;40:179–187. doi: 10.1016/j.canep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 130.Fan Z., Chen L., Li J., Cheng X., Yang J., Tian C., Zhang Y., Huang S., Liu Z., Cheng J. Clinical features of COVID-19-related liver functional abnormality. Clin. Gastroenterol. Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M., Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun J., Aghemo A., Forner A., Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 134.Tapper E.B., Asrani S.K. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J. Hepatol. 2020:1–5. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boeckmans J., Rodrigues R.M., Demuyser T., Piérard D., Vanhaecke T., Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch. Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Genovese M.C., Kremer J.M., van Vollenhoven R.F., Alten R., Scali J.J., Kelman A., Dimonaco S., Brockwell L. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheum. 2017;69:1751–1761. doi: 10.1002/art.40176. [DOI] [PubMed] [Google Scholar]
- 137.Barry A., Apisarnthanarax S., O’Kane G.M. Management of primary hepatic malignancies during the COVID-19 pandemic: recommendations for risk mitigation from a multidisciplinary perspective. Lancet Gastroenterol. Hepatol. 2020;5:765–775. doi: 10.1016/S2468-1253(20)30182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang P., Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72:124–131. doi: 10.1159/000111718. [DOI] [PubMed] [Google Scholar]
- 139.Chan S.L., Kudo M. Impacts of COVID-19 on liver cancers: during and after the pandemic. Liver Cancer. 2020;9:491–502. doi: 10.1159/000510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ng J., Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann. Transl. Med. 2016;4 doi: 10.21037/atm.2016.01.32. 118–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The immune response and immunopathology of COVID-19. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taefehshokr N., Taefehshokr S., Hemmat N., Heit B. Covid-19: perspectives on innate immune evasion. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leuchte K., Staib E., Thelen M. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Slovak R., Ludwig J.M., Gettinger S.N., Herbst R.S., Kim H.S. Immuno-thermal ablations – boosting the anticancer immune response. J. Immunother. Cancer. 2017;5:78. doi: 10.1186/s40425-017-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glassberg M.B., Ghosh S., Clymer J.W., Qadeer R.A., Ferko N.C., Sadeghirad B., Wright G.W.J., Amaral J.F. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. OncoTargets Ther. 2019;12:6407–6438. doi: 10.2147/OTT.S204340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan W., Deng Q., Lin S., Wang Y., Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int. J. Hyperther. 2019;36:263–271. doi: 10.1080/02656736.2018.1562571. [DOI] [PubMed] [Google Scholar]
- 147.Gambichler T., Reuther J., Scheel C.H., Susok L., Kern P., Becker J.C. Cancer and immune checkpoint inhibitor treatment in the era of SARS-CoV-2 infection. Cancers (Basel) 2020;12:3383. doi: 10.3390/cancers12113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kattan J., Kattan C., Assi T. Do checkpoint inhibitors compromise the cancer patients’ immunity and increase the vulnerability to COVID-19 infection? Immunotherapy. 2020;12:351–354. doi: 10.2217/imt-2020-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quaglino P., Fava P., Brizio M., Marra E., Rubatto M., Agostini A., Tonella L., Ribero S., Fierro M.T. Metastatic melanoma treatment with checkpoint inhibitors in the COVID-19 era: experience from an Italian Skin Cancer Unit. J. Eur. Acad. Dermatol. Venereol. 2020;34:1395–1396. doi: 10.1111/jdv.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmidle P., Biedermann T., Posch C. COVID-19 in a melanoma patient under treatment with checkpoint inhibition. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.