Figure 1.
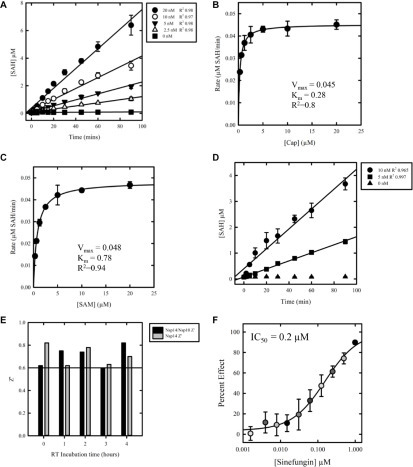
Enzyme kinetics and assay development. Standard curves were used to determine the concentration of SAH produced by reaction. (A) Reaction time course up to 90 min to determine initial rates. Nsp14 was tested at 20, 10, 5, and 2.5 nM; 20 µM was the concentration for both SAM and cap. (B) Determination of apparent KM (KMapp) for cap with nsp14. Nsp14 was tested at 10 nM; the cap was titrated from 20 to 0 µM for 60 min. The results are representative of three independent experiments. (C) Determination of apparent KM (KMapp) for SAM with nsp14. Nsp14 was tested at 10 nM; SAM was titrated from 20 to 0 µM for 60 min. The results are representative of three independent experiments. (D) Determination of the incubation time range and nsp14 concentration using KMapp for both cap and SAM. Nsp14 was tested at 10 and 5 nM. (E) Comparison of reagent stability following preincubation at a range of time points prior to the assay, with or without the addition of nsp10. The impact on assay stability was determined by comparison of Z′. (F) Dose–response testing of sinefungin against nsp14 using established screening conditions. Compound was tested at 10 concentrations, 1:2 from 100 µM (top concentration). Data are shown as mean ± SD. The IC50 value was 0.2 µM (0.1–0.3 µM; n = 4).
