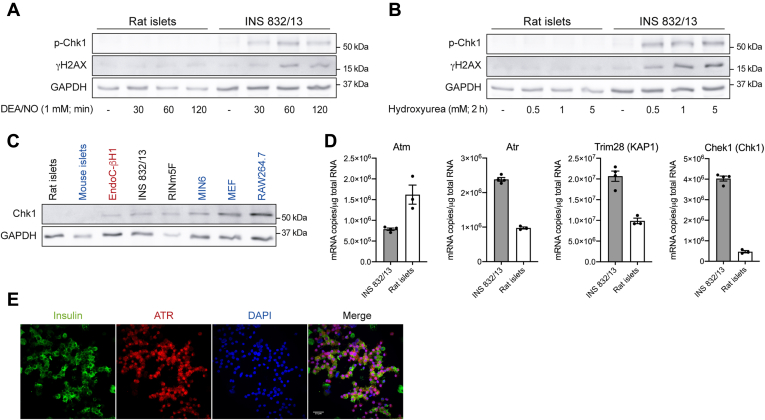
Figure 10.
Effects of nitric oxide on ATR signaling in islets. Rat islets and INS 832/13 cells (A and B) were treated with DEA/NO for the indicated times (A) or treated for 2 h with hydroxyurea (B). The cells were harvested, and the phosphorylation of Chk1 and H2AX was determined by Western blot. GAPDH levels were determined to control for protein loading. The levels of Chk1 in rodent islets and various immortalized β-cell lines (EndoC-βH1, INS 832/13, RINm5F, and MIN6) and non–β-cell lines (MEF and RAW 264.7) were determined by Western blot analysis (C). The steady state levels of Atm, Atr, Trim28, and Chek1 mRNA in INS 832/13 cells and rat islets were quantified by quantitative RT-PCR (D). Islet cell expression of ATR was determined by immunofluorescence (E) of dispersed rat islet cells stained for insulin (green), ATR (red), and nuclei (DAPI, blue). Cells were visualized using Nikon Eclipse Ti2-E microscope equipped with a Yokogawa confocal scanner unit (CSU-W1) (60× with 2× field zoom). Results are representative (A, B, C, and E) or the average ± SEM (D) of two to four independent experiments. ATR, ataxia–telangiectasia and Rad3-related protein; Chk1, checkpoint kinase 1; DAPI, 4′,6-diamidino-2-phenylindole; DEA/NO, 2-(N,N-diethylamino)-diazenolate-2-oxide; H2AX, H2A histone family member X; MEF, mouse embryonic fibroblast.