Figure 2.
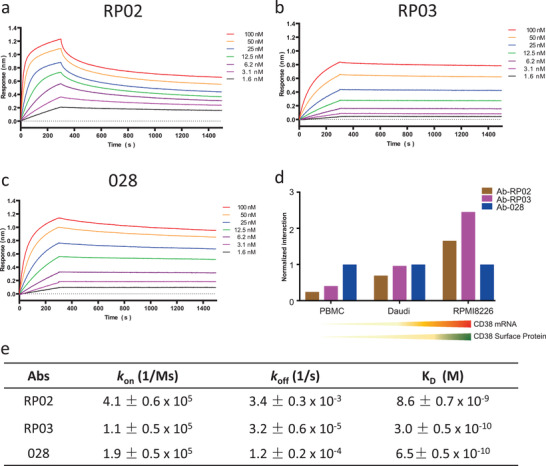
Binding affinity of anti‐CD38 scFv antibodies. a–c) Binding kinetics of anti‐CD38 RP02, RP03, and 028 scFv antibodies against purified hCD38‐ECD were investigated by biolayer interferometry. The dissociation constants were 8.6, 0.3, and 0.65 × 10−9 m for RP02, RP03, and 028, respectively. d) Purified scFv antibodies selectively bind to tumor cells with different CD38 expression levels. ScFv antibodies were incubated with hPBMC, Daudi, or RPMI8226 cells and stained with fluorescence labeled secondary antibody. The mean fluorescence intensity (MFI) of each antibody with different cells was normalized to the MFI of 028 scFv antibody with the corresponding cells. The mRNA and cell surface expression levels of CD38 are indicated below; the detailed CD38 expression analyses are shown in Figure S4 (Supporting Information). e) Kinetic rates and binding constants were obtained by fitting the binding curves generated for individual curves to the 1:1 interaction model.
