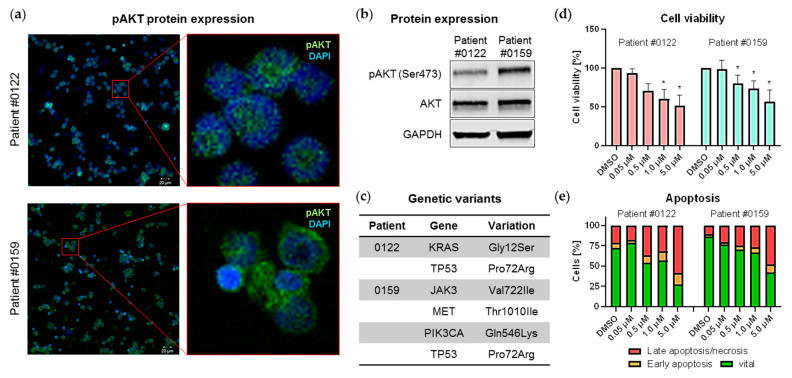
Figure 4.
Effects of MK-2206 on primary B-ALL blasts. (a,b) Protein expression of phosphorylated and total AKT was measured by immunofluorescence staining (a) and immunoblotting (b). Cells of patients #0122 and #0159 were orthotopically xenografted into NSG mice as described in [33,34] and spleen cells were subsequently harvested for further analysis. (a) Expression of pAKT was determined by immunofluorescence using the LSM780 confocal microscope (Zeiss) at 20-fold magnification (left panel). The right hand images are enlargements of the regions indicated in the left hand red boxes. (b) Cells were lysed and analyzed for phosphorylated and total AKT protein expression by immunoblot. GAPDH was used as internal loading control. Blots were processed and cropped using Image Studio Lite 5.2 software and MS PowerPoint (2011) to improve clarity and conciseness. (c) Genetic variants in both patients were detected using Cancer hotspot panel (Ion PGM System, Thermo Fisher Scientific) and previously described in [34]. (d,e) Xenograft-derived primary blasts were cultured on a murine stromal cell feeder layer system and cell viability and apoptosis induction were assessed after 72 h incubation with increasing concentrations of MK-2206. (d) Cell viability was determined by CellTiter Blue assay in technical triplicates. Mean ± standard deviation of three individual biological replicates. Significance was determined by student’s t test after testing Gaussian distribution. * P < 0.05. (e) Cells were stained with anti-human CD19, annexin V-FITC and PI for apoptosis analysis by flow cytometry. Cells positive for annexin V and negative for PI are indicated as early apoptotic whereas cells positive for both markers are considered late apoptotic/necrotic. Human primary blasts and murine feeder cells were discriminated by anti-human CD19 staining.