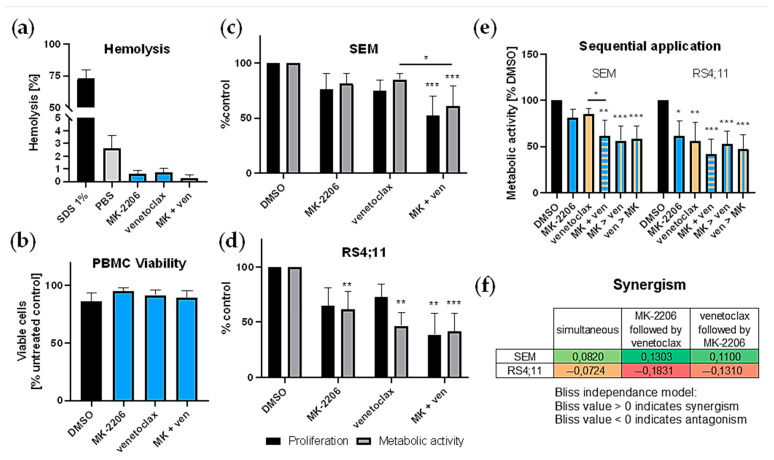
Figure 5.
Combined application of MK-2206 (MK) and venetoclax (ven). (a,b) Cytotoxicity of MK-2206 (0.25 µM), venetoclax (10 nM) or the combination was determined by hemolysis assay and PBMC viability analysis. Blood of five healthy voluntary donors was used. Technical triplicates were performed for both assays. Mean ± standard deviation. (a) Hemolytic activity was assessed by hemoglobin release after 120 min incubation with the substances, 1% SDS (positive control) or PBS (negative control). (b) PBMCs were isolated by density gradient centrifugation and incubated with the respective inhibitors or 10 µM DMSO (control) for 24 h. Cell viability was determined by Calcein AM assay. (c,d) Assessment of cell proliferation and metabolic activity was performed by trypan blue staining and WST-1 assay, respectively and compared to DMSO-treated control cells. Cells were incubated with 0.25 µM MK-2206, 10 nM (SEM (c)) or 2.5 nM (RS4;11 (d)) venetoclax or both inhibitors for 72 h. (e) Effects of sequential application of MK-2206 and venetoclax on SEM and RS4;11 cells were determined by WST-1 assay. Substances were applied either simultaneously (MK + ven) or the second substance was added 24 h after the first (MK > ven or ven > MK). Metabolic activity was assessed after 72 h. Mean ± standard deviation of at least three individual biological replicates. Significance was determined by one-way ANOVA. * P < 0.05; ** P < 0.01; *** P < 0.001. Asterisks demonstrate significance compared to DMSO-treated control cells or other treatments when connected by lines. (f) Synergy was calculated according to the Bliss independence model with values > 0 indicating synergistic combinations [35]. Bliss values were calculated based on the mean biological response of at least four biological replicates. Cells were incubated with MK-2206, venetoclax or both (simultaneously or sequentially) and metabolic activity was assessed by WST-1 assay after 72 h.