Fig. 6.
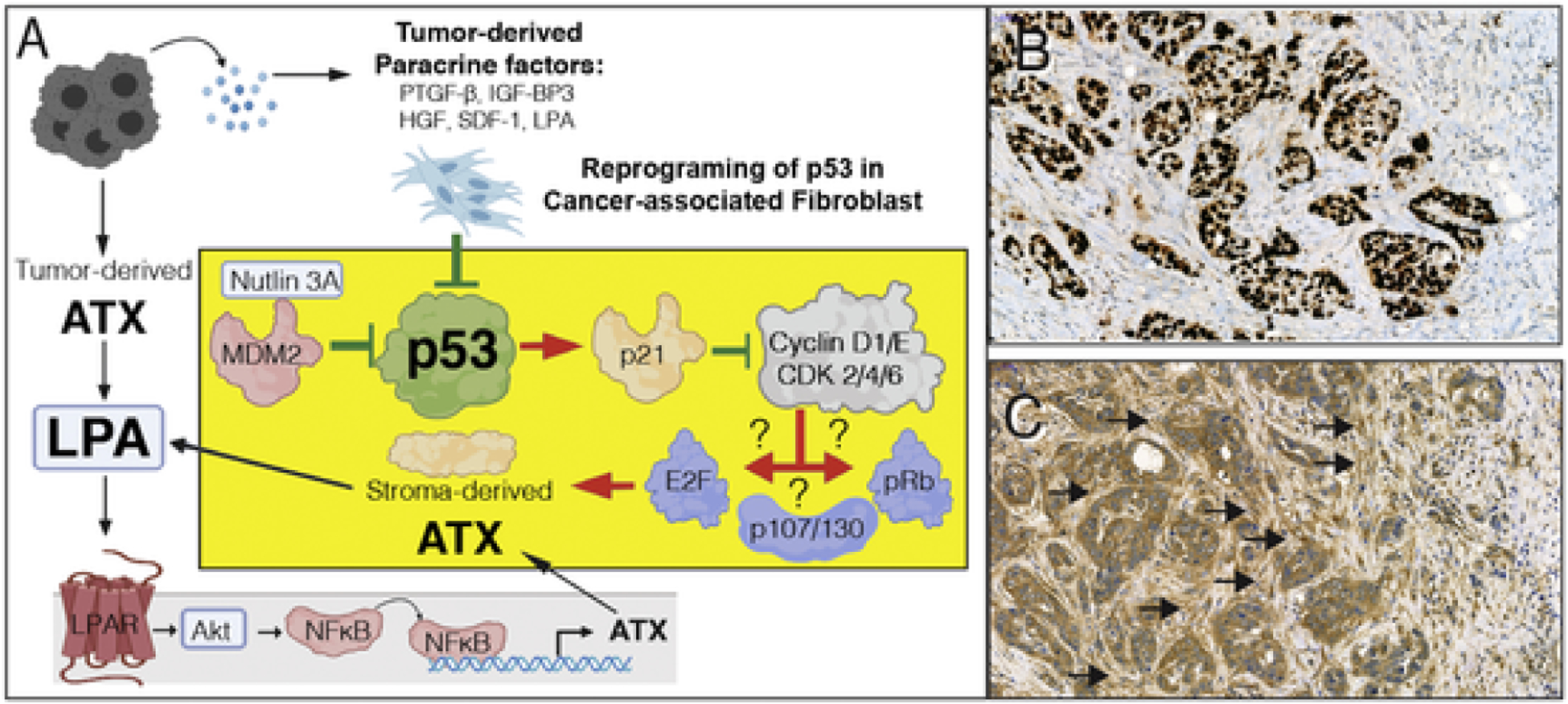
A. Hypothesis for p53 inhibition-mediated upregulation of ATX in CAF. Tumor cell derived paracrine factors inhibit p53, which in turn abolishes p21-mediated inhibition of downstream signals that include cyclinD1/E and cyclin-dependent kinase 2/4/6. Dysinhibition of the cyclin-CDK axis further activates downstream targets such as E2F, p107/130, and retinoblastoma (Rb) transcription factors. It is yet to be determined which of transcription factors is responsible for the upregulation of ATX transcription. On the other hand, tumor cell-derived ATX via the LPAR pathway has been demonstrated to drive ATX expression via NFkB-mediated transcriptional activation. Red arrows represent activation, green lines represent inhibition. The yellow block represents a CAF and the suggested mechanism for p53 inactivation and the ensuing activation of p53 transcription. B & C. A representative ATX immunostaining of an infiltrating ductal breast carcinoma case stained with anti-p53 (B) and 4F1 anti-ATX rat monoclonal antibody (C). Note that the tumor cells are intensively stained for p53, an indication that it is stabilized due to mutation. An adjacent section shows strong desmoplastic reaction with stromal fibroblasts in the immediate vicinity of the tumor cells displaying strong ATX positivity whereas, stromal cells farther away from the tumor (upper right corner of the panel) do not express ATX. Black arrows denote clusters of ATX positive CAF. De-identified specimens exempted from IRB regulation were obtained from the UTHSC Tissue Archive.
