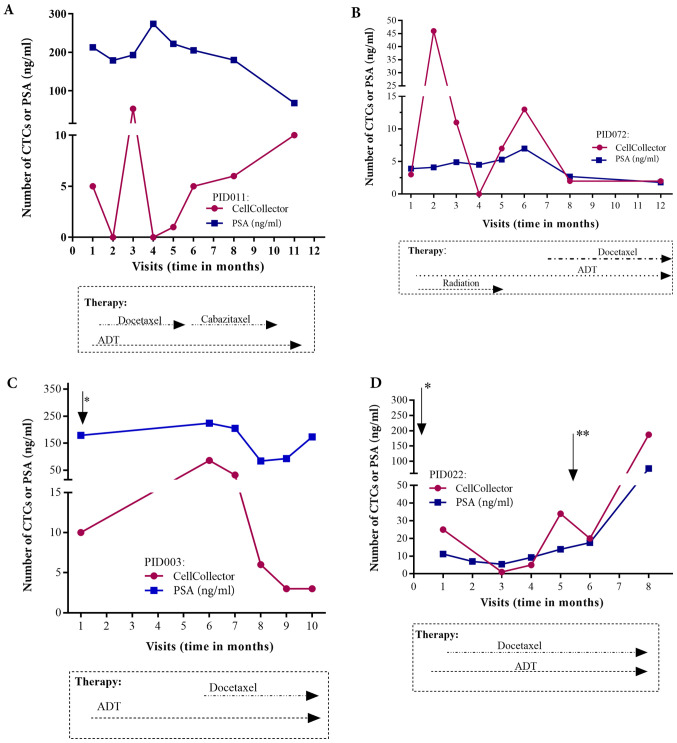
Figure 6.
Treatment history and CTC/PSA profiles of 4 metastasized PCa patients, which fulfill the inclusion criteria, up to 12 months. (A) Patient enrolled in the study 4 months after palliative TURP (B) Patient with highly differentiated adenocarcinoma 18 years after prostatectomy. (C) Patient included one month after palliative TURP (*) and showing continuous hormone therapy and disease progression. (D) Only 6 days after study inclusion, palliative TURP (*) was performed with continuous hormone therapy and radiotherapy and removal of a brain metastasis between months 5 and 6 (**). CTCs, circulating tumor cells; PSA, prostate specific antigen; PCa, prostate cancer; TURP, transurethral resection of the prostate.