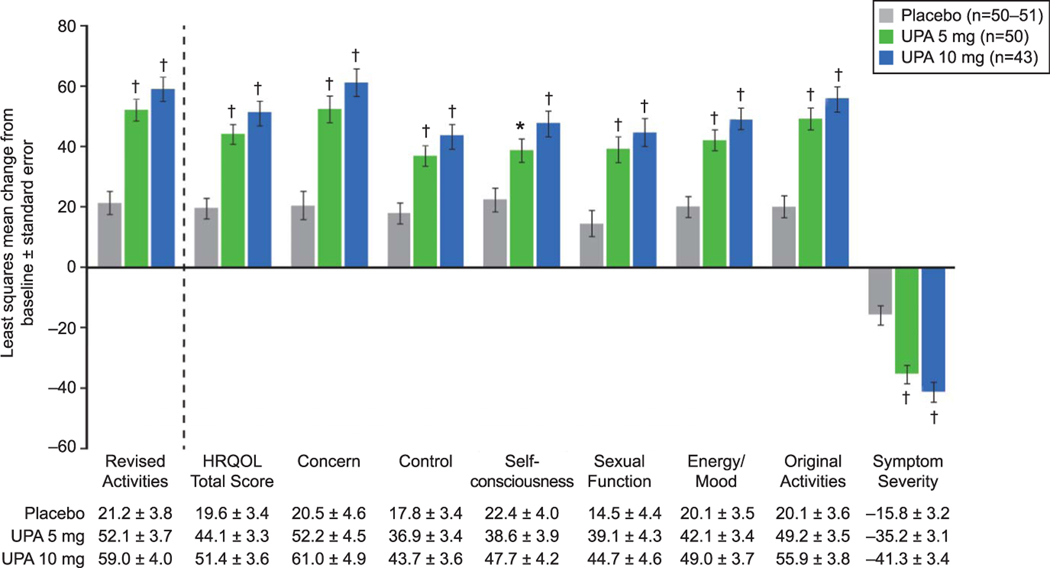
Fig. 4.
Change from baseline at end of treatment in Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire (UFS-QOL) scores (intent-to-treat population). *P<.01, †P<.001 vs placebo. Error bars represent standard error. Analysis of covariance controlling for baseline score and pooled study site was used for change from baseline in UFS-QOL scores. Health-Related Quality of Life (HRQOL) Total score and HRQOL subscale scores range from 0 to 100; higher scores indicate better HRQOL. Symptom severity subscale score ranges from 0 to 100; higher scores indicate greater symptom severity. UPA, ulipristal acetate.