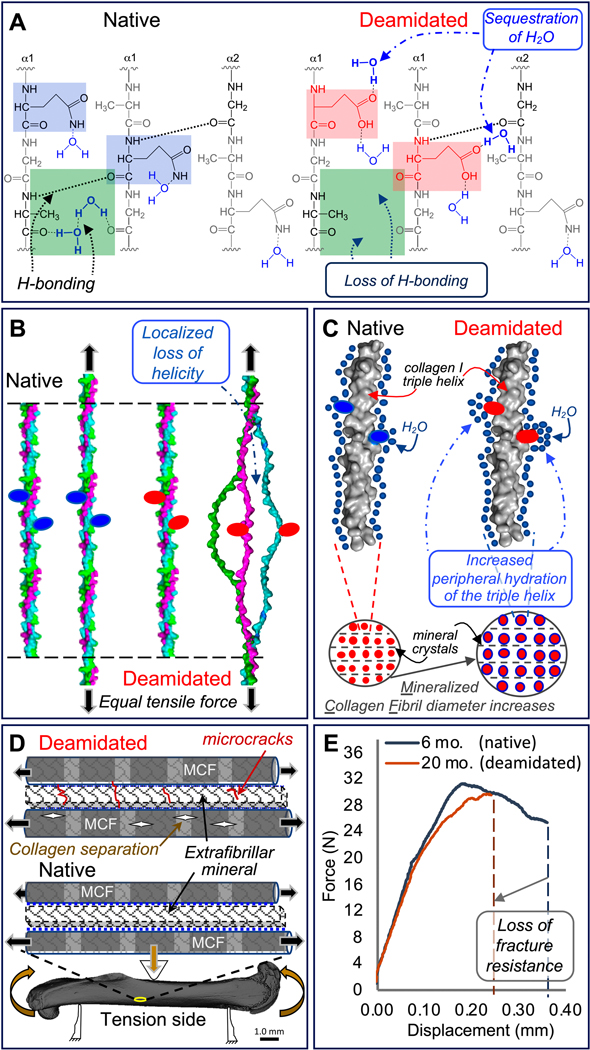
Figure 5. Proposed mechanism for how deamidation affects bone toughness.
Because deamidated sites (pink squares) sequester water molecules altering hydrogen-bonding within the collagen I triple helix (green squares), the stability of the triple helix possibly declines (A). With less stability arising from a loss of optimal hydrogen bonding, the triple helix unravels in the vicinity of deamidated sites (red ovals) at lower tensile forces compared to the native triple helix (B). Deamidation increases local hydration (blue circles) of the triple helix surface and periphery. This results in “swelling” of the mineralized collagen fibrils (MCF) and an increase in MCF diameter (C). Both lower triple helix stability and higher MCF diameter increase the likelihood of collagen ruptures within the MCF and microcrack accumulation in the extrafibrillar mineral when a bone is loaded through the yield point of the tissue (D). As a result, deamidated bone has less capacity than native bone to deform before fracture as depicted by force vs. displacement curves from three-point bending tests of femurs from 6-mo. and 20-mo. old BALB/c mice (E).