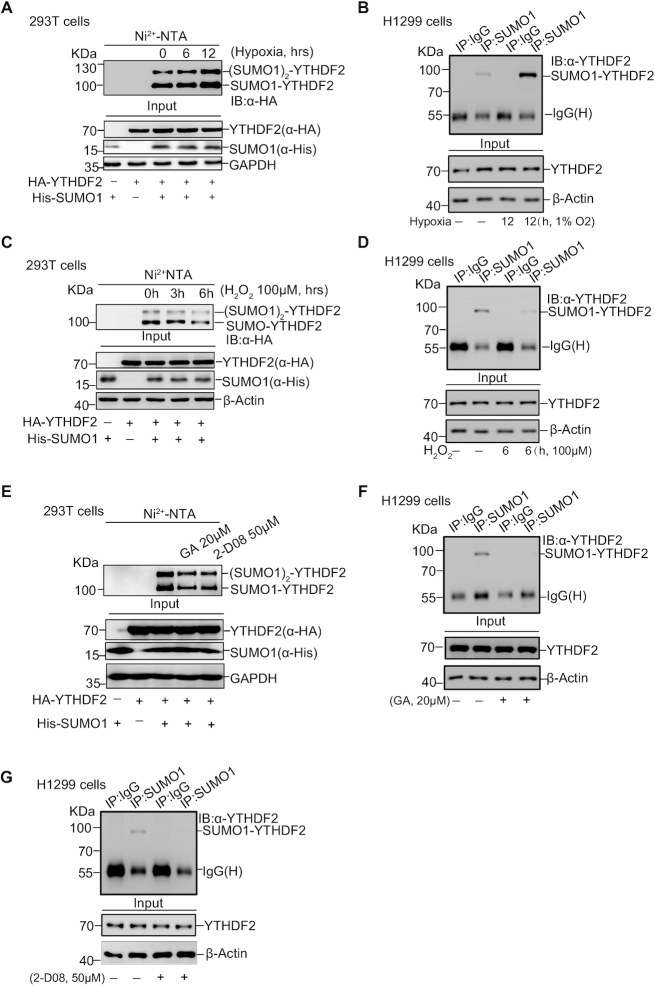
Figure 2.
SUMOylation of YTHDF2 is regulated by hypoxia, oxidative stress and SUMO inhibitors. (A, B) Hypoxia upregulates SUMOylation of YTHDF2. HEK-293T cells were transfected with HA-YTHDF2 and His-SUMO1 and treated with hypoxia (1% O2) for the indicated time. SUMO1 conjugated proteins were enriched by Ni2+-NTA resin, and then immunoblotted with anti-HA antibody (A). SUMOylation of endogenous YTHDF2 under hypoxia treatment for 12 hours was detected by IP with anti-SUMO1 and immunoblotted with anti-YTHDF2 antibody in H1299 cells (B). (C, D) Hydrogen peroxide (H2O2) downregulates SUMOylation of YTHDF2. HEK-293T cells were transfected with HA-YTHDF2 and His-SUMO1, and treated with H2O2 (100 μM) as indicated time. The Ni2+-NTA resin pull down assay was performed to detect YTHDF2 SUMOylation (C). H1299 cells treated H2O2 (100 μM) for 6 h were lysed for detection of endogenous YTHDF2 SUMOylation by using the IP/WB method as Figure 2B (D). (E–G) SUMOylation inhibitors (Ginkgolic acid and 2-D08) decrease YTHDF2 SUMOylation. HEK-293T cells were transfected with HA-YTHDF2 and His-SUMO1, and treated with Ginkgolic acid (GA, 20 μM) or 2-D08 (50 μM) for 12 or 24 h, respectively. SUMOylation of YTHDF2 was detected by Ni2+-NTA resin pull-down assay (E). SUMOylation of endogenous YTHDF2 in H1299 cells treated with 20 μM GA for 12 h (F) or 50 μM 2-D08 for 24 h (G) was detected by IP with anti-SUMO1 and immunoblotting with anti-YTHDF2 antibody.