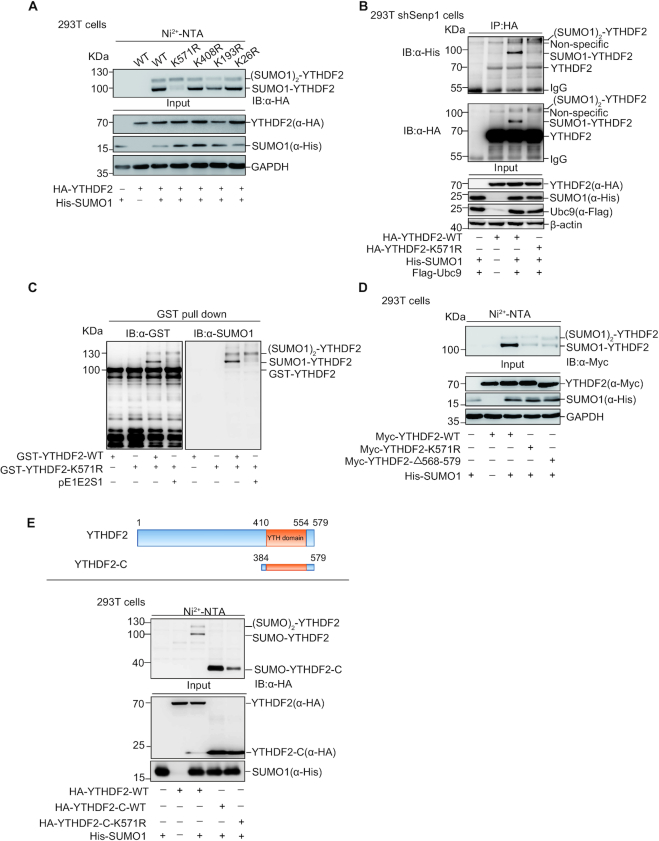
Figure 3.
YTHDF2 is mainly SUMOylated at K571. (A) Point mutants of YTHDF2 with His-SUMO1 were transfected into HEK-293T as indicated. SUMO1 conjugated proteins enriched by Ni2+-NTA resin, and eluates were immunoblotted with anti-HA antibody. (B) HEK-293T shSenp1 cells were transfected as indicated constructs. Cell lysates were IP with anti-HA antibody, and then immunoblotted with anti-HA and anti-His antibodies. (C) Mutation of K571R impairs YTHDF2 SUMOylation in the E.coli SUMOylation system. The construct pGEX-4T-1-YTHDF2 or pGEX-4T-1-YTHDF2-K571R was transformed into E. coli BL21 (DE3) with or without pE1E2SUMO1. Bacteria lysates were incubated with GST resin, and then eluates were immunoblotted with anti-GST and anti-SUMO1 antibodies. (D) Deletion of C-terminus (aa 569–579) abolishes YTHDF2 SUMOylation, as like mutant YTHDF2-K571R. His-SUMO1 with Myc-YTHDF2, Myc-YTHDF2-K571R, or Myc-YTHDF2-Δ568–579 was transfected into HEK-293T cells. SUMO1 conjugated proteins were enriched by Ni2+-NTA resin, and eluates were immunoblotted with anti-HA antibody. (E) The truncated C-terminal form, mutant K571R and full length of YTHDF2 with His-SUMO1 were transfected into HEK-293T cells. The Ni2+-NTA resin pull down assay was performed to detect SUMOylation of YTHDF2.