Figure 2.
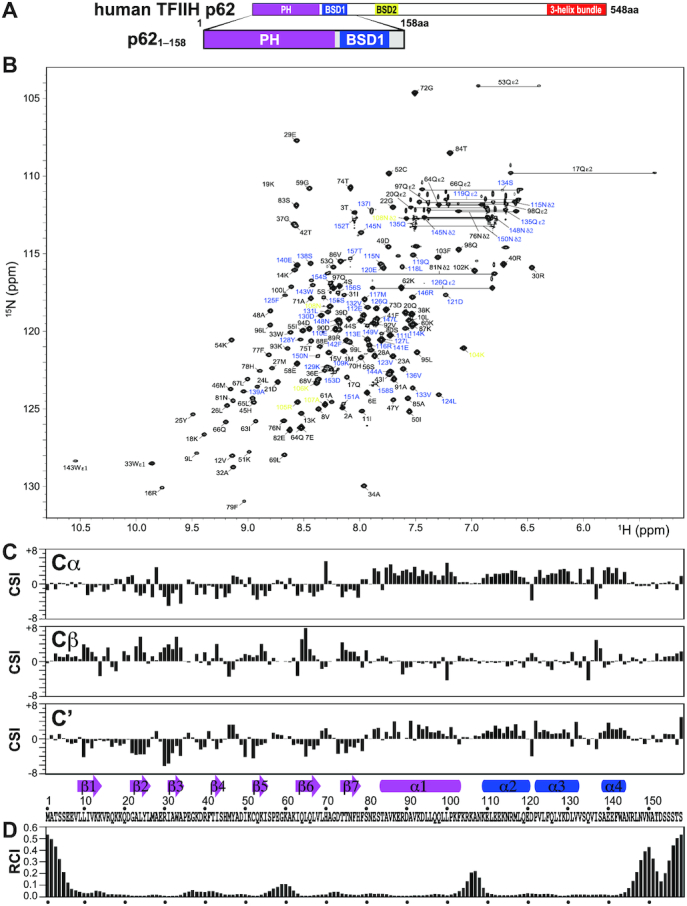
NMR signal assignment and secondary structure of the PH and BSD1 domains of human TFIIH p62. (A) p621–158 construct used in the NMR experiments. (B) 1H–15N-HSQC spectrum. Residues 1–103, 104–108 and 109–158 are labeled in black, yellow and blue, respectively. (C) Chemical shift index (CSI) of 13Cα, 13Cβ and 13C′, and identified secondary structure elements. Arrow: β-strand; rectangle; α-helix. The secondary structure identification was performed using the CSI 2.0 (http://csi.wishartlab.com) web server (79). (D) Random coil index (RCI), calculated by using the difference between observed and reference random coil shifts of 13Cα, 13Cβ, 13C′, 15N and 1H. RCI should be proportional to the flexibility of the protein backbone (80).
