Figure 6.
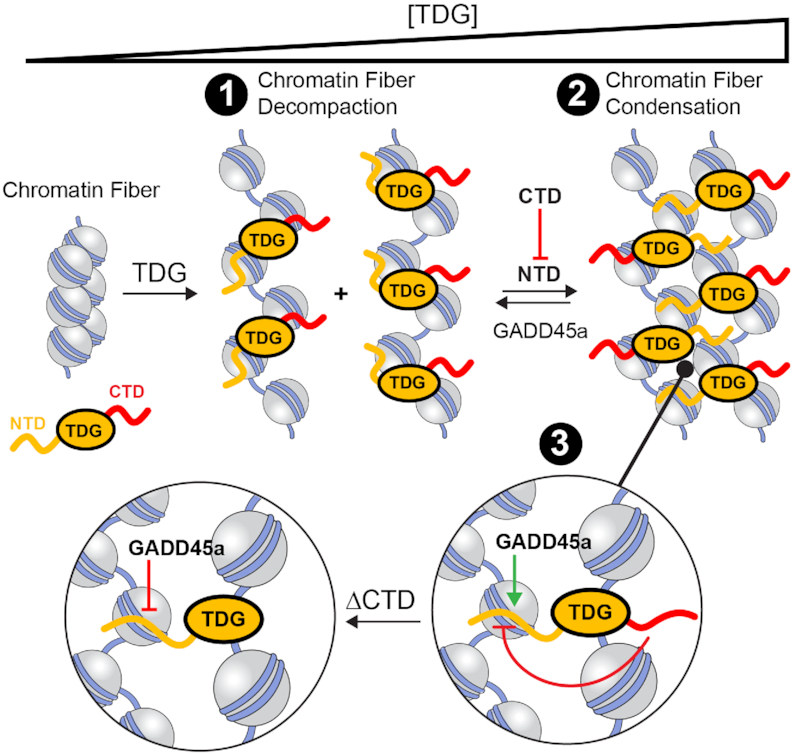
Proposed model for TDG-mediated chromatin remodelling (1). Upon recruitment, TDG preferentially binds to linker DNA between nucleosome resulting in decompaction of the chromatin fiber structure. The basic NTD contributes to nonspecific DNA binding in cis (i.e. to the same fiber as the catalytic domain) (37,38). (2) In the presence of nearby chromatin fibers, TDG’s NTD can also bind to DNA in trans (i.e. to a different fiber than the catalytic domain), facilitating oligomerization and condensation of the chromatin as local concentration of TDG increase. Because efficient oligomerization requires tethering of the NTD to chromatin (Figure 3C), we propose that DNA binding by the catalytic domain (which requires in cis DNA binding by the NTD), along with accompanying array decompaction, precedes oligomerization. (3) The CTD of TDG antagonizes chromatin condensation by weakening inter-fiber interactions between the NTD and DNA, potentially through direct contacts between the two disordered domains (38). This destabilizing affect allows for external regulators (e.g. GADD45a) to bind to and sequester TDG’s NTD away from DNA, resulting in disruption of inter-fiber interactions and re-solubilization of the chromatin. However, in the absence of the CTD’s destabilizing affect (ΔCTD), chromatin condensation becomes non-reversible due to tight inter-fiber binding of the NTD.
