Figure 5.
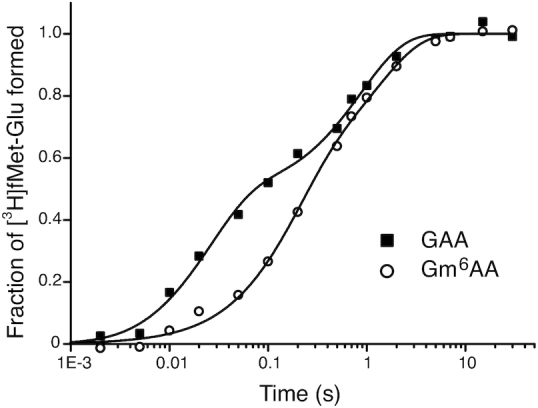
Competition between GTPase deficient and wild type T3 for native and m6A modified A-site codon. Fraction of fMet-Glu formed (y-axis) at different reaction times (x-axis, log10 display) in experiments where free T3 with wild type EF-Tu competes with GTPase deficient H84A-mutated EF-Tu for ribosomes with GAA (closed squares) and Gm6AA (open circles) codons. Both types of ternary complex are rapidly added to ribosomal complex RC2 (Figure 1) leading to rapid peptide bond formation upon initial native T3 binding and slow peptide bond formation upon initial GTPase deficient T3 binding and its eventual exchange for native T3. Note that peptide bond formation is much slower at Gm6AA than GAA codons due to m6A-dependent reduction of the rate constant for binding of T3 to A site (see main text).
