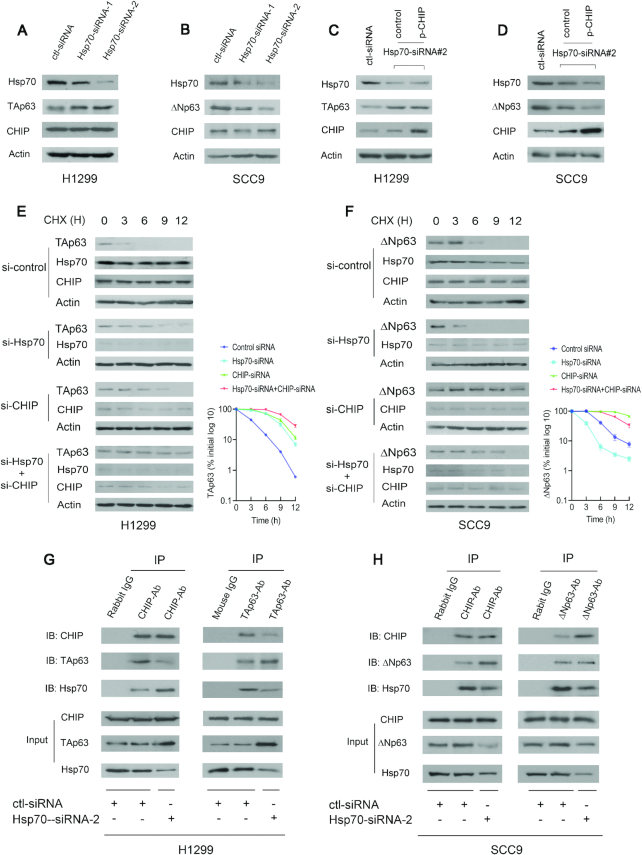
Figure 4.
Hsp70 is a molecular switch that regulates CHIP-mediated p63 degradation. (A) H1299 cells were transfected with Hsp70-siRNA-1 or Hsp70-siRNA-2 and analyzed by western blotting with Hsp70-specific (C92F3A5), TAp63-specific (Poly 618902), CHIP-specific (H-231) antibodies. Actin expression was examined as a loading control. (B) Similar to (A), except that SCC9 cells and ΔNp63-specific (Poly 619002) antibody were used. (C) H1299 cells were transfected with Hsp70-siRNA2 or control-siRNA. 30 h later, the cells were further transfected with the CHIP expression plasmid and analyzed by western blotting using the indicated antibodies. (D) Similar to (C), except that SCC9 cells and ΔNp63-specific (Poly 619002) antibody were used. (E) H1299 cells were transfected with control-siRNA, or Hsp70-siRNA2 (si-Hsp70), or CHIP-siRNA4 (si-CHIP), or both Hsp70-siRNA2 and CHIP-siRNA4, and followed by treatment with cycloheximide (CHX) (20 μg/ml). The half-life of endogenous TAp63 was measured by western blotting with TAp63-specific (Poly 618902) antibody at the indicated time-points. The protein levels of TAp63 expression were determined by densitometry and plotted (right image). Errors bars indicate the SEM (n = 3). (F) Similar to (E), except that SCC9 cells were used. The half-life of endogenous ΔNp63 was measured by western blotting with ΔNp63-specific (Poly 619002) antibody. (G) Cell extracts prepared from H1299 cells treated with control-siRNA or Hsp70-siRNA-2 were immunoprecipitated with anti-CHIP (H-231) or anti-TAp63 (Poly 618902) antibodies and immunoblotted with anti-CHIP (C10), anti-TAp63 (938102) and anti-Hsp70 (C92F3A5) antibodies. Direct western blots for CHIP, TAp63 and Hsp70 are shown in the lower panels. (H) Similar to (G), except that SCC9 cells and anti-ΔNp63-specific (Poly 619002) antibody were used as indicated.