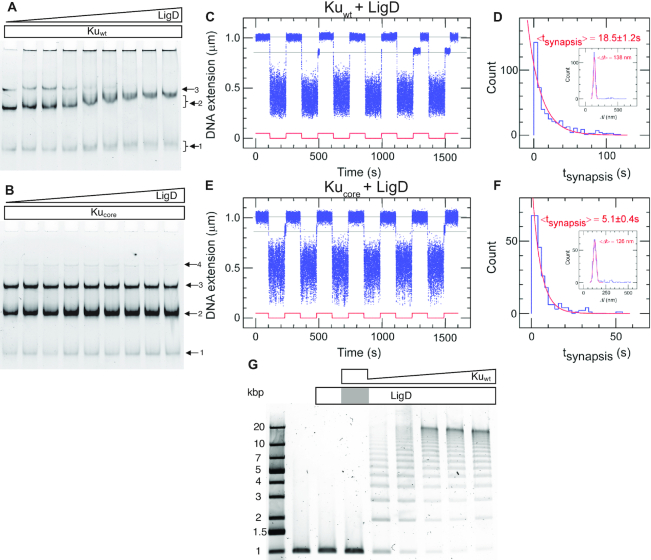
Figure 3.
The C-terminal arms of Ku are required for recruitment of LigD and full stabilization of the pre-ligation complex. EMSA gel of a 50 bp blunt-ended DNA substrate incubated with 0.2 μM (A) Kuwt and (B) Kucore and an increasing concentration of LigD (0–0.8 μM, 0.1 μM increments). The arrows indicate the number of Ku homodimers loaded on the DNA substrate. (C) Time-trace of molecular forceps experiment (blue), where a blunt-ended DNA substrate is used together with Kuwt (5 nM) and LigD (5 nM). End-to-end synapses were detected upon repeated force cycling (red). (D) Lifetime distribution of the specific end-binding events is fit to a single-exponential, giving a mean lifetime of 18.5 ± 1.2 s (SEM, n = 459). End-specific events are identified (inset) as having a Δl value within three standard deviations of the mean expected amplitude change given bridge mechanics (Gaussian fit in red, <Δl> = 138 ± 15 nm, SD, n = 486). (E, F) as C and D but for Kucore, giving a mean synapsis lifetime of 5.1 ± 0.4s (SEM, n = 270). End-specific events are identified (inset) as having a Δl value within 3 standard deviations of the mean expected amplitude change given bridge mechanics (Gaussian fit in red, <Δl> = 126 ± 16 nm, SD, n = 305). (G) Ligation of a 1000 bp blunt-ended DNA substrate with a constant amount of LigD (0.1 μM) and increasing concentration of Kuwt (0–1 μM, 0.2 μM increments). The grey area in the LigD bar indicates the absence of LigD in the corresponding sample, where only Ku is added at a concentration of 1 μM.