Figure 8.
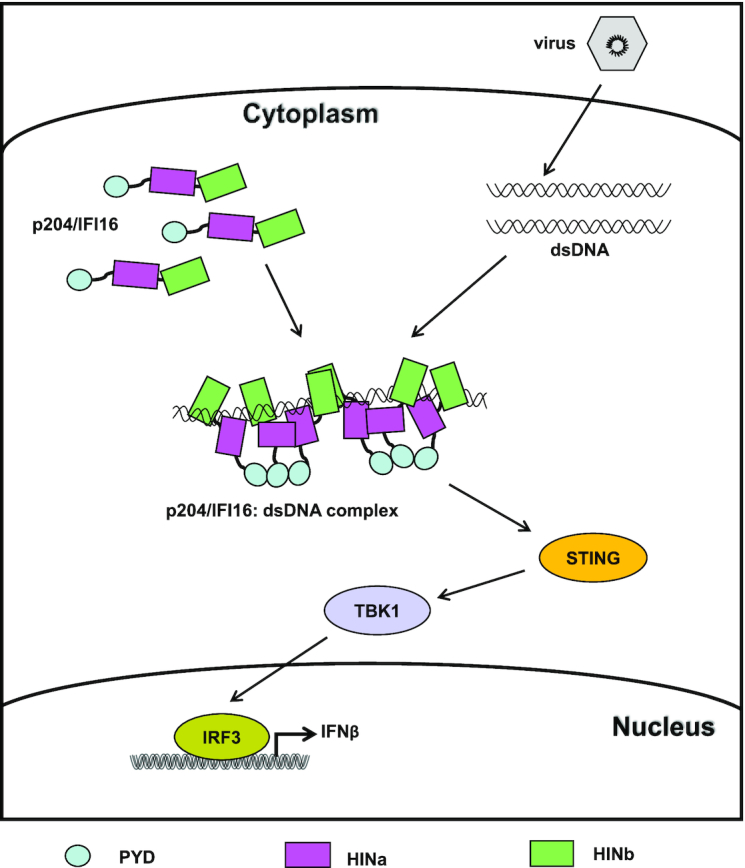
A working model of IFI16/p204 recognizing dsDNA to active downstream signaling pathway. In the absence of DNA, IFI16/p204 presents an extended conformation in the cytoplasm. Once recognizing dsDNA from invading virus, IFI16/p204 HINab domain synergistically binds to dsDNA. More HINab molecules bind to the long dsDNA and form a C-ring shaped structure around dsDNA. The binding of dsDNA stabilizes the dimerization of HINa domain, resulting in the adjacent N-terminal PYD domain are closer and aggregate to activate STING. Activated STING leads to the phosphorylation of TBK1 and IRF3 and induces the production of IFNβ and other proinflammatory cytokines to defend against the virus infection.
