Abstract
Objective
To evaluate systematically the efficacy and safety of COVID-19 vaccines.
Methods
PubMed, Embase, Cochrane Library, Clinicaltrial.gov, CNKI, Wanfang Data, China Biomedical Literature Service System, and China Clinical Trial Registry were searched for randomized controlled trials of COVID-19 vaccines published up to December 31, 2020. The Cochrane bias risk assessment tool was used to assess the quality of studies. A qualitative analysis was performed on the results of clinical trials.
Results
Thirteen randomized, blinded, controlled trials, which involved the safety and efficacy of 11 COVID-19 vaccines, were included. In 10 studies, the 28-day seroconversion rate of subjects exceeded 80%. In two 10 000-scale clinical trials, the vaccines were effective in 95% and 70.4% of the subjects, respectively. The seroconversion rate was lower than 60% in only one study. In six studies, the proportion of subjects who had an adverse reaction within 28 days after vaccination was lower than 30%. This proportion was 30%-50% in two studies and > 50% in the other two studies. Most of the adverse reactions were mild to moderate and resolved within 24 hours after vaccination. The most common local adverse reaction was pain or tenderness at the injection site, and the most common systemic adverse reaction was fatigue, fever, or bodily pain. The immune response and incidence of adverse reactions to the vaccines were positively correlated with the dose given to the subjects. The immune response to the vaccines was worse in the elderly than in the younger population. In 6 studies that compared single-dose and double-dose vaccination, 4 studies showed that double-dose vaccination produced a stronger immune response than single-dose vaccination.
Conclusions
Most of the COVID-19 vaccines appear to be effective and safe. Double-dose vaccination is recommended. However, more research is needed to investigate the long-term efficacy and safety of the vaccines and the influence of dose, age, and production process on the protective efficacy.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Systematic review, Efficacy, Safety, Clinical trial
Abstract
目的
系统评价新型冠状病毒肺炎(COVID-19)疫苗的有效性和安全性。
方法
通过计算机检索有关COVID-19疫苗的临床随机对照试验文献,对临床试验结果进行定性分析。检索时间为各数据库建库至2020年12月31日。所检索的数据库包括PubMed、Embase、Cochrane图书馆、Clinicaltrial.gov、中国知网、万方数据、中国生物医学文献服务系统和中国临床试验注册中心。使用Cochrane偏倚风险评估工具评估文献质量。
结果
纳入了13项随机、盲法、对照试验,涉及11种COVID-19疫苗接种的安全性和有效性。在其中10项研究中,受试者的28 d血清转化率超过80%;2项万人规模的临床试验中,分别取得了95%和70.4%的有效率;1项研究的血清转化率低于60%。在对接种后28 d内不良反应发生率的分析显示,6项研究不良反应发生率低于30%,2项研究为30%~50%,2项研究高于50%。在13项研究中,疫苗接种不良反应事件绝大部分为轻度到中度,在接种后24 h内缓解;最常见的局部不良反应为注射部位疼痛或压痛,最常见的系统性不良反应为疲劳、发热或躯体痛。受试者对疫苗的免疫反应和不良反应发生率与接种剂量呈正相关。老年人对疫苗的免疫反应较年轻人差。6项研究比较了疫苗单剂量与双剂量接种的效应,其中4项研究显示双剂量接种比单剂量接种产生更强的免疫反应。
结论
大部分COVID-19疫苗具有较好的有效性和安全性;推荐双剂量接种。然而COVID-19疫苗的长期有效性、安全性及剂量、年龄和工艺差异对保护效力的影响需要更多的研究证实。
Keywords: 新型冠状病毒肺炎, 严重急性呼吸综合征冠状病毒2, 疫苗, 系统评价, 有效性, 安全性, 临床试验
It has been more than a year since the outbreak of the novel coronavirus pneumonia (COVID-19). Although the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused COVID-19 in China was effectively controlled, the global epidemic has not stopped. According to data from the World Health Organization, as of 16:05 on February 15, 2021, Central European Time, the cumulative number of confirmed COVID-19 cases worldwide reached 108, 579, 352, and the cumulative deaths reached 2, 396, 408[1]. The COVID-19 epidemic as a major global public health event has become the primary health threat for all mankind, and impacted the world's political, economic and cultural greatly[2-3]. SARS-CoV-2 is a β-coronavirus with RNA as genetic material, which enters cell through a spike protein combined with angiotensin converting enzyme 2[4-5]. COVID-19 generally manifests as fever and dry cough, and injuries multiple organ, especially the lungs[2, 5-6]. Wearing mask and maintaining social distancing have been confirmed as the most effective measures to stop the spread of the virus form China's experience of fighting the epidemic[3, 7-9], and isolation and symptomatic supportive treatment still dominate for COVID-19 patients[5]. However, the efficacy of antiviral drugs and traditional Chinese medicines needs more evidence[10-11]. Due to the low penetration rate of masks and the limitations of treatment options abroad[12-13], more and more hopes are pinned on the development of a COVID-19 vaccine. According to different targets and technologies, vaccines can be divided into the following categories: inactivated vaccines, recombinant spike protein vaccines, viral vector vaccines, RNA vaccines, live attenuated vaccines and virus-like particle vaccines, etc[14-16]. Currently, hundreds of COVID-19 candidate vaccine projects have been registered in the US clinical trial database (clinicaltrials.gov)[15, 17]. Results of phase 3 clinical trials of several vaccines are published[18-22]. As of January 1, 2021, China, Russia, the United States, Britain and other countries have approved their own mass vaccination plans for the population. This study evaluated the safety and effectiveness of the COVID-19 vaccine through systematic literature review and qualitative analysis for the published COVID-19 vaccine clinical trial results.
1. Information and methods
This systematic review was completed in accordance with the guidelines in the "Preferred Reporting Project for Systematic Evaluation and Meta-Analysis (PRISMA)"[23-24].
1.1. Literature inclusion criteria
The literature inclusion criteria: (1) The healthy men or non-pregnant women aged 18 and above; (2) COVID-19 vaccination as the intervention measure; (3) The randomized, controlled, and blinded trials; (4) The clinical trial results indicators include at least one or more as following: local adverse reactions (pain, itching, redness, swelling and induration, etc.), systemic adverse reactions (fever, diarrhea, fatigue, nausea/vomiting, etc.), the last vaccine neutralizing antibody geometric mean titer (GMT), seroconversion rate and other laboratory test indicators measured by live virus neutralization test 14 days or 28 days after inoculation.
1.2. Literature exclusion criteria
Documents that meet one of the following conditions were excluded: (1) Medical news, popular science articles, non-medical papers, reviews, letters, comments, basic research, case reports, conference abstracts; (2) No full text or literature published in a third language other than Chinese and English; (3) One of overlapping two studies were excluded; (4) If the data of the literature included in the later published literature, The former was excluded.
1.3. Literature search
The English databases PubMed, Embase, Cochrane Library and clinicaltrials.gov databases were searched. The Chinese databases searched included CNKI, Wanfang Database, China Biomedical Literature Service System and China Clinical Trial Registration Center. In order to ensure the comprehensiveness of the search results, this system evaluation used Boolean logic to search by "subject words + free words". The main search terms include: COVID-19, 2019-nCoV, SARS-CoV-2, 2019 novel coronavirus, vaccines, vaccination, COVID-19 vaccines, mRNA-1273 vaccine, Ad5-nCoV vaccine, ChAdOx1 COVID-19 vaccine, BNT162 vaccine, controlled clinical trial, randomized controlled trials, controlled clinical trial, random, blind, placebo, trial, Meta, and etc. Chinese search terms include: 新型冠状病毒、新冠肺炎、新型冠状病毒肺炎、疫苗、试验、随机对照试验、随机对照研究、随机对照、随机、元分析、Meta、荟萃, etc.
1.4. Literature screening and data extraction
The literature screening and data extraction were done independently by two researchers. Differences in the summary of the results will be discussed and dealt with by the two researchers or the third researcher. All results obtained in the database were imported into Note Express (Wuhan University Library Edition) software, and duplicate documents were removed mechanically using the software's duplicate check function. The initial screening by reading the title and abstract, and the secondary screening by reading the full text were completed. The extracted data included: the first author, vaccine type, inoculation dose, interval between inoculations, number of subjects and baseline characteristics (race, sex ratio, age range or average age), research design, local and systemic adverse reactions, laboratory indicators, as well as funds, sponsors and registration number.
1.5. Methodological quality evaluation
Assess the risk of bias according to the Cochrane Systematic Review Manual[25-26].
1.6. Statistical analysis
The main results of this systematic review included the safety and effectiveness of the vaccine. Indicators for evaluating safety included local adverse reactions (pain, itching, redness, induration, etc.) and systemic adverse reactions (cough, diarrhea, fatigue, fever, headache, nausea/vomiting, itching, muscle pain, joint pain/discomfort, anorexia, etc.). The immunogenicity indicators included GMT, seroconversion rate, and the response of IgG or other specific antibodies to the receptor binding domain.
2. Results
2.1. Literature search results
There were 753 relevant articles published before December 31, 2020. After screening, 13 papers were included in the system evaluation[19-22, 27-35]. The process of document screening was shown in Figure 1.
Figure 1.
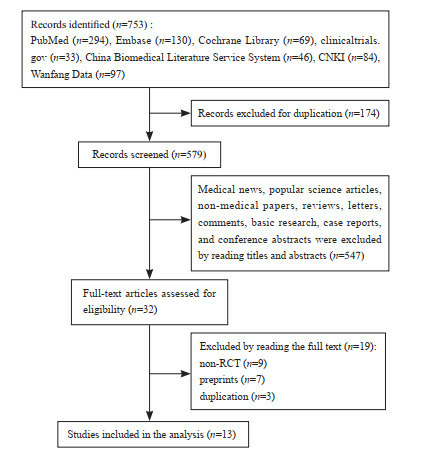
A flow diagram of literature screening
2.2. Methodological quality evaluation
The 13 included studies all adopted a randomized control method[19-22, 27-35], with a double-blind method in 10 studies[21-22, 27-32, 34-35], and a single-blind method in 2 studies[20, 33], and bothsingle-blind method and double-blind methodin one study[19]. All trials hid the allocation plan. Nine trials had incomplete data or selective reports[19, 22, 27, 29-31, 33-35], of which 2 had more missing data in the preprint[22, 29], and the remaining 7 missed individual data[19, 27, 30-31, 33-35]; 9 trials had other types of bias[19-20, 22, 29-32, 34-35], for example, Keech et al.[30] did not perform virus neutralization test in the experimental design. In general, the included literature had a low risk of bias (Figure 2 & Table 1).
Figure 2.
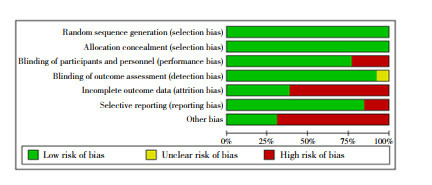
Risk assessment of literature bias
Table 1.
Methodological quality evaluation of included studies
| Studies | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Voysey 2021[19] | low risk | low risk | high risk | low risk | high risk | low risk | high risk |
| Polack 2020[20] | low risk | low risk | high risk | low risk | low risk | low risk | high risk |
| Xia 2020[21] | low risk | low risk | low risk | low risk | low risk | low risk | low risk |
| Pu 2020[22] | low risk | low risk | low risk | low risk | high risk | low risk | high risk |
| Xia 2021[27] | low risk | low risk | low risk | low risk | high risk | low risk | low risk |
| Che 2020[28] | low risk | low risk | low risk | low risk | low risk | low risk | low risk |
| Ella 2020[29] | low risk | low risk | low risk | low risk | high risk | low risk | high risk |
| Keech 2020[30] | low risk | low risk | low risk | unclear | high risk | low risk | high risk |
| Mulligan 2020[31] | low risk | low risk | low risk | low risk | high risk | low risk | high risk |
| Richmond 2020[32] | low risk | low risk | low risk | low risk | low risk | low risk | high risk |
| Walsh 2020[33] | low risk | low risk | high risk | low risk | high risk | low risk | low risk |
| Zhang 2021[34] | low risk | low risk | low risk | low risk | high risk | high risk | high risk |
| Zhu 2020[35] | low risk | low risk | low risk | low risk | low risk | high risk | high risk |
2.3. The characteristics of the included studies
The 13 included studies were randomized, blinded, and controlled trials, involving 5 inactivated vaccines[21-22, 27-29, 34], 2 recombinant spike protein vaccines[30, 32], 2 RNA vaccines[20, 31, 33] and 2 adenovirus vector vaccines[19, 35]. Table 2 for details of vaccine characteristics and developer information). There were 6 studies comparing the effects of single-dose and double-dose vaccination[19, 27, 30-31, 33, 35]. Most of the 13 studies compared the difference of two doses of vaccine at intervals of 2, 3 or 4 weeks. Most studies also compared the difference between low, medium and high injection doses. Participants in all trials were adults, and 5 articles reported the results of vaccines in the elderly population[19-20, 32-33, 35]. The baseline characteristics of the participants were shown in Table 3.
Table 2.
Experimental design and developers of the included studies
| Studies | Vaccines | Adjuvant | Research type | Phase | Developers | Registration ID |
| Voysey 2021[19] | Adenovirus recombinant vector vaccine (ChAdOx1 nCoV-19/AZD1222) | No | Randomized double /single blind control | I/II/III | AstraZeneca | NCT04324606, NCT04400838, NCT04444674 |
| Polack 2020[20] | RNA vaccine (BNT162b2) | Lipid nanoparticle | Randomized single-blind control | II/III | BioNTech / Pfizer | NCT04368729 |
| Xia 2020[21] | Inactivated vaccine | Aluminum hydroxide | Randomized double-blind control | I/II | Wuhan Institute of Biological Products Co. Ltd | ChiCTR2000031809 |
| Pu 2020[22] | Inactivated vaccine | Aluminum hydroxide | Randomized double-blind control | I | Institute of Medical Biology, Chinese Academy of Medical Sciences | NCT04412538 |
| Xia 2021[27] | Inactivated vaccine (BBIBP-CorV) | Aluminum hydroxide | Randomized double-blind control | I/II | Beijing Institute of Biological Products | ChiCTR2000032459 |
| Che 2020[28] | Inactivated vaccine | Aluminum hydroxide | Randomized double-blind control | II | Institute of Medical Biology, Chinese Academy of Medical Sciences | NCT04412538 |
| Ella 2020[29] | Inactivated vaccine (BBV152) | Algel-IMDGor Algel | Randomized double-blind control | I | Bharat Biotech | NCT04471519 |
| Keech 2020[30] | Recombinant spiroprotein nanoparticle vaccine (NVX-CoV2373) | Mareix-m1 | Randomized double-blind control | II | Novavax | NCT04368988 |
| Mulligan 2020[31] | RNA vaccine (BNT162b1) | Lipid nanoparticle | Randomized double-blind control | I/II | BioNTech/Pfizer | NCT04368728 |
| Richmond 2020[32] | Recombinant spiroprotein vaccine (SCB-2019) | ASO3 or CpG/Alum | Randomized double-blind control | I | Clover Biopharmaceuticals | NCT04405908 |
| Walsh 2020[33] | RNA vaccine (BNT162b1/ BNT162b2) | Lipid nanoparticle | Randomized single-blind control | I | BioNTech/ Pfizer | NCT04368728 |
| Zhang 2021[34] | Inactivated vaccine | Aluminum hydroxide | Randomized double-blind control | I/II | SINOVAC BIOTECH CO.LTD. | NCT04352608 |
| Zhu 2020[35] | Adenovirus type-5-vectored vaccine | No | Randomized double-blind control | II | Beijing Institute of Biotechnology and Citic Biological | NCT04341389 |
Table 3.
Baseline characteristics of the participants
| Studies | Age (years) | Male/Female (n) | Experimental group/control group (n) | Injected dose | Injection procedure*** | Country/ethnic group |
| * The study did not report a mean age; # The median age; ** Only the data of subjects without any evidence of SARS-CoV-2 infection before vaccination were selected; *** The numbers in parentheses indicate when vaccine was injected, for example (0, 28) means that the vaccine is injected again on the 28th day after the first injection. | ||||||
| Voysey 2021[19] | ≥18* | 55, 447/54, 360 | 55, 048/54, 759 | 2.2×1010, (3.5-6.5) ×1010 or (5-7.5)×1010 virus particles | Single injection or (0, 28) | Brazilian, South African and British/White |
| Polack 2020[20] | 52#/≥16 | 19, 129/18, 394 | 19, 198/18, 325** | 30 μg | (0, 21) | American, Argentinian, Brazilian, South African, German, Turkish/White |
| Xia 2020[21] | 41.2/18-59 | 120/200 | 240/80 | 2.5 μg, 5 μg or 10 μg | (0, 14), (0, 28) or (0, 56) | Chinese/Asian |
| Pu 2020[22] | 18-59* | unclear | 144/48 | 50 EU, 100 EU or 150 EU | (0, 14) or (0, 28) | Chinese/Asian |
| Xia 2021[27] | 53.7/≥18 | 301/339 | 470/170 | 2 μg, 4 μg or 8 μg | (0, 28) | Chinese/Asian |
| Che 2020[28] | 41.4/18-59 | 258/486 | 595/149 | 100 EU or 150 EU | (0, 14) or (0, 28) | Chinese/Asian |
| Ella 2020[29] | 18-55* | unclear | 297/73 | 3 μg or 6 μg | (0, 14) | Indian/unclear |
| Keech 2020[30] | 30.8/18-59 | 63/62 | 102/23 | 5 μg or 25 μg | (0, 21) | Australian /White |
| Mulligan 2020[31] | 35.4/18-55 | 23/22 | 36/9 | 10 μg, 30 μg or 100 μg | Single injection or (0, 21) | German/ White |
| Richmond 2020[32] | 35.7/18-75 | 70/78 | 118/30 | 3 μg, 9 μg or 30 μg | (0, 21) | Australian/White |
| Walsh 2020[33] | 35.9/18-85 | 94/101 | 156/39 | 10 μg, 20 μg, 30 μg or 100 μg | Single injection or (0, 21) | American/White |
| Zhang 2021[34] | 42.6/18-59 | 345/389 | 568/166 | 3 μg or 6 μg | (0, 14) or (0, 28) | Chinese/Asian |
| Zhu 2020[35] | 39.7/≥18 | 445/445 | 382/508 | 5×1010 or 1×1011 virus particles | Single injection | Chinese/Asian |
2.4. Qualitative analysis
2.4.1. The effectiveness and safety of vaccines
In 10 studies, the 28-day seroconversion rate of testee exceeded 80%[21-22, 27-34]. The RNA vaccine (BNT162b2) reported by Polack achieved 95% efficiency[20], the recombinant adenovirus vector vaccine (ChAdOx1 nCoV-19) reported by Voysey achieved an effective rate of 70.4%[19], but Zhu reported that the 28-day seroconversion rate of the adenovirus recombinant vector vaccine in testee was less than 60%[35].
In 6 studies, the incidence of adverse reactions in volunteers within 28 days for vaccination was less than 30%[20-22, 27-28, 34]. The adverse reaction rates of the recombinant spike protein vaccine (SCB-2019) reported by Richmond[32] and the RNA vaccine reported by Walsh[33] were 34.7% and 39.1%, respectively, and the adverse reaction rates of the RNA vaccine (BNT162b1) reported by Mulligan[31] and the adenovirus recombinant vector vaccine reported by Zhu[35] were 52.8% and 73.0%, respectively. Three studies could not obtain the adverse reaction rate[19, 29-30]. The adverse reactions of all vaccinated testee were mostly mild to moderate, and could be relieved within 24 hours after vaccination. The most common local adverse reaction included pain or tenderness at the injection site[19-22, 27-35]. Fatigue was reported as the most common systemic adverse reaction in 9 studies[19-20, 22, 28-29, 31, 33-35]. In addition, fever was reported as the most common systemic adverse reaction in 2 studies[21, 27], and 2 studies reported somatic pain as the most common systemic adverse reaction[30, 32] (Table 4).
Table 4.
Effectiveness and safety of vaccines
| Studies | Key effectiveness indicators | Total incidence of adverse reactions [%(n/N)) | Incidence of serious adverse reactions [%(n/N)) | The most common adverse reactions | |
| Local reactions | Systemic reactions | ||||
| GMT: geometric mean titers; * The efficacy is calculated from the corrected relative risk; ** Efficacy =100×(1-IRR), IRR is the ratio of the number of confirmed COVID-19 cases per 1000 person-years of follow-up in the vaccine group to the corresponding cases in the placebo group; # The original literature only gave the incidence of adverse reactions, but did not give the specific number of people. | |||||
| Voysey 2021[19] | Efficacy 70.4%* | Unclear | 0.15 (84/55, 048) | Pressing pain | fatigue |
| Polack 2020[20] | Efficacy 95%** | 27.0%# | Unclear | Pain | Fatigue |
| Xia 2020[21] | Day 14 seroconversion rates: 97.6% in the middle dose group; Day 14 GMT: 121 in the standard dose group | 15.0(36/240) | 0(0/240) | Pain | Fever |
| Pu 2020[22] | Day 28 seroconversion rates: 80%, 96% and 92% in the low dose, middle dose and high dose groups respectively; Day 28 GMT: 10.6, 15.4 and 19.6 in the low dose, middle dose and high dose groups respectively | 25.7(37/144) | 0(0/144) | Pain | Fatigue |
| Xia 2021[27] | Day 28 seroconversion rates: 100% each in the low dose, middle dose and high dose groups; Day 28 GMT: 13.4, 18.9 and 23.7 in the low dose, middle dose and high dose groups respectively | 29.2(42/144) | 0(0/144) | Pain | Fever |
| Che 2020[28] | Day 28 seroconversion rates: 92% in the middle dose group and 96% in the high dose group; Day 28 GMT: 19 in the middle dose group and 21 in the high dose group | 24.5(146/595) | 0(0/595) | Pain | Fatigue |
| Ella 2020[29] | Day 28 seroconversion rates: 87.9% in the low dose group and 91.9% in the high dose group; Day 28 GMT: 61.7 in the low dose group and 66.4 in the high dose group | Unclear | Unclear | Pain | Fatigue |
| Keech 2020[30] | Day 35 GMT: 4-6 times higher than that of serum in convalescent period | Unclear | 1.96(2/102) | Pressing pain | Arthralgia |
| Mulligan 2020[31] | Day 28 GMT: 168 in the low dose group and 267 in the middle dose group | 52.8(19/36) | 5.6(2/36) | Pain | Fatigue |
| Richmond 2020[32] | Day 36 seroconversion rates: 95%, 100% and 100% in the low dose, middle dose and high dose groups respectively | 34.7(41/118) | 1.69(2/118) | Pain | Headache |
| Walsh 2020[33] | Day 28 GMT (BNT162b1 vaccine): 168, 167 and 267 in the low dose, middle dose and high dose groups respectively; Day 28 GMT (BNT162b2 vaccine): 157, 263 and 361 in the low dose, middle dose and high dose groups respectively | 39.1(61/156) | 4.49(7/156) | Pain | Fatigue |
| Zhang 2021[34] | Day 28 seroconversion rates: 25% in the low dose group and 83% in the high dose group; Day 28 GMT: 5.4 in the low dose group and 15.2 in the high dose group | 26.6(151/568) | 1.04(1/96) | Pain | Fatigue |
| Zhu 2020[35] | Day 28 seroconversion rates: 59% in the low dose group and 47% in the standard dose group; Day 28 GMT: 18.3 in the low dose group and 19.5 in the standard dose group | 73.0(279/382) | 6.5(25/382) | Pain | Fatigue |
2.4.2. Dose difference
The difference in injection dose is an important factor affecting the immunogenicity and safety of the vaccine. A total of 9 studies[21-22, 27-29, 32-35] found significant differences in GMT and seroconversion rates obtained from testee with different doses of vaccination, 8 of which[20-22, 28-29, 31, 34-35] found that GMT increased, and 4[22, 28-29, 32] found that the seroconversion rate of testee increased with the increase of vaccine dose, but the incidence of adverse reactions also increases relatively [22, 28-29, 32]. Therefore, when the clinical trial entered Phase III, the researchers set the medium dose as the standard dose of the vaccine [19-20].
2.4.3. Difference of age
Four studies specifically recruited the elderly 60 years and older, and conducted a special subgroup analysis in the results. Richmond [32] reported that the GMT range measured by the micro-neutralization test in the elderly group was 1567-3625, which was lower than 2510-4452 in the 18-59-year-old group. The incidence of systemic adverse reactions in the elderly after the first injection was 17%, which was lower than 38% in the 18-59 years-old group. Xia [27] also reported that the GMT of the elderly group was lower than that of the 18-59 years-old group, and the seroconversion time was later than that of the 18-59 years-old group. The incidence of systemic adverse reactions in the elderly within 7 days after vaccination was 28.6%, which was lower than 41.7% of the 18-59 years-old group. Polack[20] and Walsh[33] also reported similar results. In short, compared with healthy people aged 18 to 59, the GMT detected in the serum was significantly lower in elderly population vaccinated with the same vaccine according to the same procedure, but the incidence of adverse reactions in the elderly population was also significantly lower [20, 27, 32-33].
2.4.4. Differences in vaccination procedures
Although a number of studies designed a comparison of different vaccination procedures, the results of the experiment were complicated. Zhang 's research showed that testee who vaccinated at 2-week intervals got a faster immune response, but a stronger immune response at 4-week intervals[34]. Che detected a stronger immune response in testee who were vaccinated at 2-week intervals[28]. Xia also found that the incidence of adverse reactions in testee vaccinated at 2-week intervals was lower than that at 4-week intervals[21]. In 6 studies that compared single-dose and double-dose vaccination, 4 studies showed that double-dose vaccination produced a stronger immune response than single-dose vaccination[19, 31, 33, 35].
2.4.5. Differences of vaccine type
The RNA vaccine (BNT162b2) reported by Polack[20] and the recombinant adenovirus vector vaccine (ChAdOx1 nCoV-19) reported by Voysey[19] involved more than 10, 000 people, and two both used relative risk to calculate the effective rate, showing that effective rate of the former was 95%[20], and the latter was 70.4%[19]. Owing to differences in the design, the small sample size, and different outcome indicators of other clinical trials, their effective rates were not yet comparable.
3. Discussion
The system evaluation draws the following conclusions: (1) All candidate vaccines have a good immunogenicity and safety except the vaccine reported by Zhu[35]. Within 28 days after vaccination, the testee' serum GMT increased significantly, and the seroconversion rate was mostly greater than 80%. The adverse reaction rate of most vaccines was less than 30%, degree was mild to moderate, and symptoms were alleviated within 24 hours. (2) The potency and adverse reaction rate after vaccination were positively related to the dose. Most clinical trials chose the middle dose when the phase III. This might be the result of comprehensive consideration of effectiveness and safety. (3) Under the same conditions, the vaccine had poor immunogenicity to elderly people over 60, but the adverse reaction rate was also low. One of the possible reasons was low immunity of the older. A lot of studies on the tolerance of the elderly population to the vaccine still are needed. In addition, there are currently no published results of clinical trials targeting juveniles. (4) Most studies recommend double-dose vaccination, but the interval needs further study.
However, this systematic review has some limitations: (1) No evidence of the long-term effectiveness and safety of the vaccine. Due to the urgency of vaccine development, most trials only followed up to 28 days after vaccination. Whether neutralizing antibodies can be maintained for a long time and whether there are delayed adverse reactions after vaccination still require a longer period. (2) In order to get more up-to-date evidence, this systematic review also includes preprinted documents, which have not been peer reviewed and some of the data are not available. (3) Only randomized, double-blind, and controlled trials were included, while observational studies, retrospective case analysis, and early animal experiments were all excluded. For example, an open label trial conducted by Anderson[36] found that mRNA-1273 vaccine had a good safety in the elderly population. Logonov[37] reported two adenovirus recombinant vector vaccine preparations (rAd26) in a non-random clinical trial (rAd26-S and rAd5-S) had a good safety and immunogenicity in healthy people aged 18 to 60. (4) There were differences in the design of various clinical trials, which made it impossible to compare the advantages and disadvantages of different types of vaccines. For example, Voysey[19] and Polack[20] used relative risk to calculate the effective rate. Although the remaining 10 studies have completed the virus neutralization test, the experimental design schemes were quite different [21-22, 27-29, 31-35]. (5) Only Chinese and English documents were searched in this systematic review, and documents published in other languages such as Japanese and French were excluded.
In conclusion, this systematic review summarized the results of clinical trials related to the COVID-19 vaccine, showing that most vaccines had a good safety and effectiveness. It is believed that with the widespread vaccination of COVID-19, it is possible to control and end the global pandemic of COVID-19.
Conflict of interest: The authors have no conflicts of interest to disclose.
新型冠状病毒肺炎(COVID-19)疫情暴发至今已1年余。虽然COVID-19疫情在我国已经得到了有效控制, 但全球整体疫情形势依然严峻。根据世界卫生组织的数据, 截至欧洲中部时间2021年2月15日16 : 05, 全球累计COVID-19确诊病例达到108 579 352例, 累计死亡人数达到2 396 408人[1]。作为全球的重大公共卫生事件, COVID-19疫情成为全人类首要的健康威胁, 世界政治经济文化也受到巨大冲击[2-3]。导致COVID-19的严重急性呼吸综合征冠状病毒2(SARS-CoV-2)是以RNA为遗传物质的β属冠状病毒, 通过刺突蛋白结合血管紧张素转化酶2进入细胞[4-5]。COVID-19患者的首发症状以发热和干咳多见, 在多脏器损伤中, 肺脏受损最为严重[2, 5-6]。在疫情控制上, 佩戴口罩和保持社交距离已经在中国抗击疫情的实践中被确认为阻断病毒传播最为有效的措施[3, 7-9]。在对COVID-19患者的治疗上, 隔离和对症支持治疗仍占主要地位[5], 而关于抗病毒药物和中药等的疗效还需更多证据的支持[10-11]。由于口罩在国外普及率的低下和治疗方案的局限性[12-13], 越来越多的希望被寄托在COVID-19疫苗的开发上。根据靶点和技术的不同, 疫苗可以被分为以下几类: 灭活疫苗、重组刺突蛋白疫苗、病毒载体疫苗、RNA疫苗、减毒活疫苗和病毒样颗粒疫苗等[14-16]。目前, 已有数百项COVID-19候选疫苗的项目在美国临床试验数据库(clinicaltrials.gov)注册[15, 17], 数种疫苗的3期临床试验结果予以发表[18-22]。截至2021年1月1日, 中、俄、美、英等国家先后批准了本国疫苗在人群中的大规模接种计划。本研究通过系统文献复习及定性分析已发表的COVID-19疫苗临床试验结果, 评估COVID-19疫苗的安全性与有效性。
1. 资料与方法
本系统评价遵循《系统评价和Meta分析的首选报告项目(PRISMA)》中的准则完成[23-24]。
1.1. 文献纳入标准
文献纳入标准包括: (1)试验对象为18岁及以上的健康男性或未孕女性; (2)干预措施为接种COVID-19疫苗; (3)试验类型为随机、对照、盲法试验; (4)临床试验结果指标至少包括以下一项或几项: 局部不良反应(疼痛、瘙痒、发红、肿胀和硬结等)、全身不良反应(发热、腹泻、疲劳、恶心/呕吐等)、末次疫苗接种14 d或28 d后以活病毒中和试验测得的中和抗体几何平均滴度(GMT)、血清转化率及其他实验室检测指标。
1.2. 文献排除标准
具备以下条件之一的文献被排除: (1)文献类型为医学新闻、科普文章、非医学类论文、综述、信件、评论、基础研究、病例报告、会议摘要; (2) 无法获取全文或以中文、英文外的第三种语言发表的文献; (3)若两项研究的受试者存在重叠, 则其中之一被排除; (4)若文献的数据被之后发表的文献包含在内, 前者予以排除。
1.3. 文献检索
对英文数据库PubMed、Embase、Cochrane图书馆和clinicaltrials.gov数据库进行了检索。检索的中文数据库包括中国知网、万方数据库、中国生物医学文献服务系统和中国临床试验注册中心。为了保证检索结果的全面性, 本系统评价运用布尔运算逻辑, 采取"主题词+自由词"方式进行了检索。主要检索词包括: COVID-19、2019-nCoV、SARS-CoV-2、2019 novel coronavirus、vaccines、vaccination、COVID-19 vaccines、mRNA-1273 vaccine、Ad5-nCoV vaccine、ChAdOx1 COVID-19 vaccine、BNT162 vaccine、controlled clinical trial、randomized controlled trials、controlled clinical trial、random、blind、placebo、trial、Meta等。中文检索词包括新型冠状病毒、新冠肺炎、新型冠状病毒肺炎、疫苗、试验、随机对照试验、随机对照研究、随机对照、随机、元分析、Meta、荟萃等。
1.4. 文献筛选和资料提取
文献筛选和资料提取工作由两位研究者独立完成。若结果汇总时出现分歧, 由两位研究者讨论处理或交由第3位研究者决定。在数据库中获得的所有检索结果导入NoteExpress(武汉大学图书馆版)软件中, 使用软件的查重功能机械地去除重复文献。然后通过阅读标题和摘要完成初次筛选, 通过阅读全文完成二次筛选。在第二次筛选中, 每一篇文献被剔除的原因均被记录。所提取数据包括: 第一作者、疫苗类型、接种剂量、接种间隔时间、受试者人数及基线特征(种族、性别比例、年龄范围或平均年龄)、研究设计方案、局部和全身不良反应、实验室检查指标, 以及基金、赞助商和注册号等。
1.5. 方法学质量评价
1.6. 统计学分析
本系统评价的主要结果包括疫苗的安全性和有效性。评估安全性的指标包括局部不良反应(疼痛、瘙痒、红肿、硬结等)及全身不良反应(咳嗽、腹泻、疲倦、发烧、头痛、恶心/呕吐、瘙痒、肌肉疼痛、关节痛/不适、厌食等)。评估免疫原性的指标包括GMT、血清转化率、IgG或其他特异性抗体对受体结合域的反应。
2. 结果
2.1. 文献检索结果
检索了截至2020年12月31日之前发表的所有相关文献, 共得到753篇。经过筛选后纳入13篇[19-22, 27-35]进入本系统评价。文献筛选的具体流程见图 1。
Figure 1.
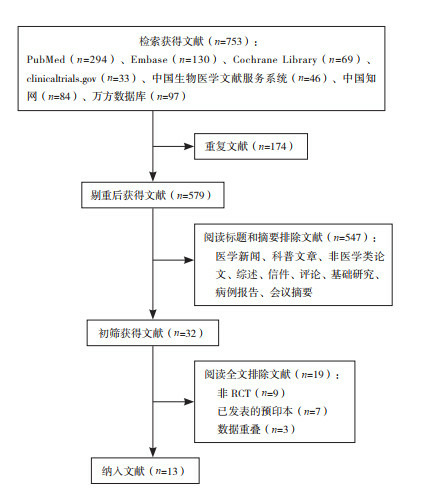
文献筛选流程图
2.2. 纳入研究的方法学质量评价
纳入的13项研究[19-22, 27-35]均采用了随机对照的方法, 其中10项[21-22, 27-32, 34-35]实施了双盲法, 2项[20, 33]实施了单盲法, 1项[19]在不同试验地点分别使用了单盲法和双盲法; 所有试验均隐藏了分配方案; 9项[19, 22, 27, 29-31, 33-35]数据不完整或选择性报告, 其中2项[22, 29]预印本缺失数据较多, 其余7项[19, 27, 30-31, 33-35]缺失个别数据; 9项[19-20, 22, 29-32, 34-35]存在其他类型偏倚, 如Keech等[30]在试验设计中未做病毒中和试验。总的来讲, 所纳入文献的偏倚风险较低。见图 2和表 1。
Figure 2.
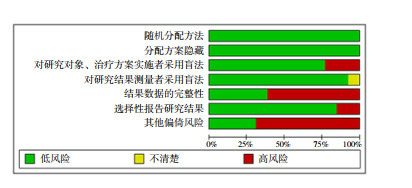
文献偏倚风险评估
Table 1.
纳入研究的方法学质量评价
| 研究 | 随机分配方法 | 分配方案隐藏 | 对研究对象、治疗方案实施者采用盲法 | 对研究结果测量者采用盲法 | 结果数据的完整性 | 选择性报告研究结果 | 其他偏倚风险 |
| Voysey 2021[19] | 低风险 | 低风险 | 高风险 | 低风险 | 高风险 | 低风险 | 高风险 |
| Polack 2020[20] | 低风险 | 低风险 | 高风险 | 低风险 | 低风险 | 低风险 | 高风险 |
| Xia 2020[21] | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 |
| Pu 2020[22] | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 低风险 | 高风险 |
| Xia 2021[27] | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 低风险 | 低风险 |
| Che 2020[28] | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 |
| Ella 2020[29] | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 低风险 | 高风险 |
| Keech 2020[30] | 低风险 | 低风险 | 低风险 | 不清楚 | 高风险 | 低风险 | 高风险 |
| Mulligan 2020[31] | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 低风险 | 高风险 |
| Richmond 2020[32] | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 |
| Walsh 2020[33] | 低风险 | 低风险 | 高风险 | 低风险 | 高风险 | 低风险 | 低风险 |
| Zhang 2021[34] | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 高风险 | 高风险 |
| Zhu 2020[35] | 低风险 | 低风险 | 低风险 | 低风险 | 低风险 | 高风险 | 高风险 |
2.3. 纳入研究的基本特征
所纳入的13项研究均为随机、盲法、对照试验, 共涉及灭活疫苗5种[21-22, 27-29, 34]、重组刺突蛋白疫苗2种[30, 32]、RNA疫苗2种[20, 31, 33]和腺病毒载体疫苗2种[19, 35], 疫苗特性、开发者等信息见表 2。有6项研究比较了疫苗单剂量与双剂量接种的效应[19, 27, 30-31, 33, 35]。大部分研究比较了以2周、3周或4周为间隔注射两剂疫苗的差别。大部分研究也比较了低、中、高不同注射剂量的差别。所有试验的参与者均为成年人, 有5篇文献报道了疫苗在老年人群体中的结果[19-20, 32-33, 35]。所纳入研究参与者的基线特征见表 3。
Table 3.
纳入研究的基线特征
| 研究 | 年龄(平均/范围, 岁) | 男/女(例) | 试验组/ 对照组(例) | 注射剂量 | 注射程序*** | 国家/主要人种 |
| 注: *该研究未报道平均年龄; #中位年龄; **仅选取了接种前无任何SARS-CoV-2感染迹象的受试者的数据; ***括号中的数字表示注射疫苗的时间, 如(0, 28)表示在第1次注射后, 第28天再次注射。 | ||||||
| Voysey 2021[19] | ≥18* | 55 447/54 360 | 55 048/54 759 | 2.2×1010、(3.5~6.5)×1010或(5~7.5)×1010病毒颗粒 | 单剂量或(0, 28) | 巴西、南非、英国/ 白人 |
| Polack 2020[20] | 52#/≥16 | 19 129/18 394 | 19 198/18 325** | 30 μg | (0, 21) | 美国、阿根廷、巴西、南非、德国及土耳其/ 白人 |
| Xia 2020[21] | 41.2/18~59 | 120/200 | 240/80 | 2.5 μg、5 μg或10 μg | (0, 14)、(0, 28)或(0, 56) | 中国/亚洲人 |
| Pu 2020[22] | 18~59* | 不详 | 144/48 | 50 EU、100 EU或150 EU | (0, 14)或(0, 28) | 中国/亚洲人 |
| Xia 2021[27] | 53.7/≥18 | 301/339 | 470/170 | 2 μg、4 μg或8 μg | (0, 28) | 中国/亚洲人 |
| Che 2020[28] | 41.4/18~59 | 258/486 | 595/149 | 100 EU或150 EU | (0, 14)或(0, 28) | 中国/亚洲人 |
| Ella 2020[29] | 18~55* | 不详 | 297/73 | 3 μg或6 μg | (0, 14) | 印度/不详 |
| Keech 2020[30] | 30.8/18~59 | 63/62 | 102/23 | 5 μg或25 μg | (0, 21) | 澳大利亚/白人 |
| Mulligan 2020[31] | 35.4/18~55 | 23/22 | 36/9 | 10 μg、30 μg或100 μg | 单剂量或(0, 21) | 德国/白人 |
| Richmond 2020[32] | 35.7/18~75 | 70/78 | 118/30 | 3 μg、9 μg或30 μg | (0, 21) | 澳大利亚/白人 |
| Walsh 2020[33] | 35.9/18~85 | 94/101 | 156/39 | 10 μg、20 μg、30 μg或100 μg | 单剂量或(0, 21) | 美国/白人 |
| Zhang 2021[34] | 42.6/18~59 | 345/389 | 568/166 | 3 μg或6 μg | (0, 14)或(0, 28) | 中国/亚洲人 |
| Zhu 2020[35] | 39.7/≥18 | 445/445 | 382/508 | 5×1010或1×1011病毒颗粒 | 单剂量 | 中国/亚洲人 |
Table 2.
纳入研究的试验设计和开发者
| 研究 | 疫苗 | 佐剂 | 研究类型 | 分期 | 开发者 | 注册号 |
| Voysey 2021[19] | 腺病毒重组载体疫苗(ChAdOx1 nCoV-19/AZD1222) | 无 | 随机双盲/ 单盲对照 | Ⅰ/ Ⅱ/ Ⅲ | AstraZeneca | NCT04324606, NCT04400838, NCT04444674 |
| Polack 2020[20] | RNA疫苗(BNT162b2) | 脂质纳米粒 | 随机单盲对照 | Ⅱ/ Ⅲ | BioNTech公司和辉瑞公司 | NCT04368729 |
| Xia 2020[21] | 灭活疫苗 | 氢氧化铝 | 随机双盲对照 | Ⅰ/ Ⅱ | 武汉生物制品研究所有限公司 | ChiCTR2000031809 |
| Pu 2020[22] | 灭活疫苗 | 氢氧化铝 | 随机双盲对照 | Ⅰ | 中国医学科学院医学生物研究所 | NCT04412538 |
| Xia 2021[27] | 灭活疫苗(BBIBP-CorV) | 氢氧化铝 | 随机双盲对照 | Ⅰ/ Ⅱ | 北京生物制品研究所 | ChiCTR2000032459 |
| Che 2020[28] | 灭活疫苗 | 氢氧化铝 | 随机双盲对照 | Ⅱ | 中国医学科学院医学生物研究所 | NCT04412538 |
| Ella 2020[29] | 灭活疫苗(BBV152) | Algel-IMDG或Algel | 随机双盲对照 | Ⅰ | Bharat生物技术公司 | NCT04471519 |
| Keech 2020[30] | 重组刺突蛋白纳米颗粒疫苗(NVX-CoV2373) | mareix-m1 | 随机双盲对照 | Ⅱ | Novavax公司 | NCT04368988 |
| Mulligan 2020[31] | RNA疫苗(BNT162b1) | 脂质纳米粒 | 随机双盲对照 | Ⅰ/ Ⅱ | BioNTech公司和辉瑞公司 | NCT04368728 |
| Richmond 2020[32] | 重组刺突蛋白疫苗(SCB-2019) | ASO3或CpG/Alum | 随机双盲对照 | Ⅰ | 三叶草生物制药 | NCT04405908 |
| Walsh 2020[33] | RNA疫苗(BNT162b1/BNT162b2) | 脂质纳米粒 | 随机单盲对照 | Ⅰ | BioNTech公司和辉瑞公司 | NCT04368728 |
| Zhang 2021[34] | 灭活疫苗 | 氢氧化铝 | 随机双盲对照 | Ⅰ/ Ⅱ | 北京科兴生物制品 | NCT04352608 |
| Zhu 2020[35] | 腺病毒5载体疫苗 | 无 | 随机双盲对照 | Ⅱ | 北京生物技术研究所和中信生物 | NCT04341389 |
2.4. 定性分析结果
2.4.1. 疫苗的有效性和安全性
在10项研究中, 受试者的28 d血清转化率超过80%[21-22, 27-34];在两项万人规模的临床试验中, Polack等[20]报道的RNA疫苗(BNT162b2)取得了95%的有效率, Voysey等[19]报道的腺病毒重组载体疫苗(ChAdOx1 nCoV-19)取得了70.4%的有效率; Zhu等[35]报道的腺病毒重组载体疫苗在受试者中的28 d血清转化率低于60%。见表 4。
Table 4.
疫苗的有效性和安全性
| 研究 | 主要有效性指标 | 总不良反应发生率[%(n/N)] | 严重不良反应发生率[%(n/N)] | 最常见的不良反应 | |
| 局部 | 系统性 | ||||
| 注: GMT为中和抗体几何平均滴度; *有效率由校正后的相对危险度计算; **有效率=100×(1-IRR), IRR为疫苗组每1 000人年随访中确诊的COVID-19病例数与安慰剂组相应病例的比率; #原文献仅给出不良反应率, 未给出具体人数。 | |||||
| Voysey 2021[19] | 有效率70.4%* | 不详 | 0.15(84/55 048) | 压痛 | 疲劳 |
| Polack 2020[20] | 有效率95%** | 27.0# | 不详 | 疼痛 | 疲劳 |
| Xia 2020[21] | 14 d血清转化率: 标准剂量组97.6%; 14 d GMT: 标准剂量组121 |
15.0(36/240) | 0(0/240) | 疼痛 | 发热 |
| Pu 2020[22] | 28 d血清转化率: 低、中、高剂量组分别为80%、96%、92%; 28 d GMT: 低、中、高剂量组分别为10.6、15.4、19.6 |
25.7(37/144) | 0(0/144) | 疼痛 | 疲劳 |
| Xia 2021[27] | 28 d血清转化率: 低、中、高剂量组均为100%;
28 d GMT: 低、中、高剂量组分别为13.4、18.9、23.7 |
29.2(42/144) | 0(0/144) | 疼痛 | 发热 |
| Che 2020[28] | 28 d血清转化率: 中剂量组92%, 高剂量组96%;
28 d GMT: 中剂量组19, 高剂量组21 |
24.5(146/595) | 0(0/595) | 疼痛 | 疲劳 |
| Ella 2020[29] | 28 d血清转化率: 低剂量组87.9%, 高剂量组91.9%;
28 d GMT: 低剂量组61.7, 高剂量组66.4 |
不详 | 不详 | 疼痛 | 疲劳 |
| Keech 2020[30] | 35 d GMT: 比恢复期血清高4~6倍 | 不详 | 1.96(2/102) | 压痛 | 关节痛 |
| Mulligan 2020[31] | 28 d GMT: 低剂量组168, 中剂量组267 | 52.8(19/36) | 5.6(2/36) | 疼痛 | 疲劳 |
| Richmond 2020[32] | 36 d血清转化率: 低、中、高剂量组分别为95%、100%、100% | 34.7(41/118) | 1.69(2/118) | 疼痛 | 头痛 |
| Walsh 2020[33] | BNT162b1疫苗28 d GMT: 低、中、高剂量组分别为168、167、267;BNT162b2疫苗28 d GMT: 低、中、高剂量组分别为157、263、361 | 39.1(61/156) | 4.49(7/156) | 疼痛 | 疲劳 |
| Zhang 2021[34] | 28 d血清转化率: 低剂量组25%, 高剂量组83%;
28 d GMT: 低剂量组5.4, 高剂量组15.2 |
26.6(151/568) | 1.04(1/96) | 疼痛 | 疲劳 |
| Zhu 2020[35] | 28 d血清转化率: 低剂量和标准剂量组分别为59%、47%;28 d GMT: 低剂量组18.3, 标准剂量组19.5 | 73.0(279/382) | 6.5(25/382) | 疼痛 | 疲劳 |
在6项研究中, 志愿者在接种疫苗后的28 d内不良反应发生率低于30%[20-22, 27-28, 34];Richmond等[32]报道的重组刺突蛋白疫苗(SCB-2019)和Walsh等[33]报道的RNA疫苗的不良反应率分别为34.7%和39.1%;Mulligan等[31]报道的RNA疫苗(BNT162b1)和Zhu等[35]报道的腺病毒重组载体疫苗的不良反应率分别为52.8%和73.0%;3项研究无法获取不良反应率[19, 29-30]。所有疫苗接种的受试者发生不良反应事件绝大部分都是轻度到中度, 且在接种后24 h内可缓解; 所有疫苗接种最常见的局部不良反应均为注射部位疼痛或压痛[19-22, 27-35];疲劳在9项研究中被报道为最常见的系统性不良反应[19-20, 22, 28-29, 31, 33-35]。此外, 发热在2项研究中被报道为最常见的系统性不良反应[21, 27], 也有2项研究报道躯体痛为最常见的系统性不良反应[30, 32]。见表 4。
2.4.2. 剂量差异的影响
注射剂量的不同是影响疫苗免疫原性和安全性的重要因素。共有9项研究[21-22, 27-29, 32-35]发现接受不同剂量疫苗接种的受试者获得的GMT和血清转化率存在显著性差异, 其中8项[20-22, 28-29, 31, 34-35]发现GMT随着疫苗剂量的增加而增加, 4项[22, 28-29, 32]发现受试者血清转化率随疫苗剂量的增加而增加。但随着接种剂量的加大, 不良反应的发生率也相对增加[22, 28-29, 32]。因此, 当临床试验进入Ⅲ期阶段, 研究者将中等剂量设定为疫苗的标准剂量[19-20]。
2.4.3. 年龄差异的影响
有4项研究专门招募了60岁及以上的老年人群, 并在结果中进行了专门的亚组分析。Richmond等[32]报道使用微量中和试验在老年人组测得的GMT范围为1 567~3 625, 低于18~59岁组的2 510~4 452;而老年人在第1次注射后的全身不良反应发生率为17%, 低于18~59岁组的38%。Xia等[27]也报道老年人组GMT低于18~59岁组, 且达到血清转化时间晚于18~59岁组; 而老年人在接种后7 d内的全身不良反应发生率为28.6%, 低于18~59岁组的41.7%。Polack等[20]和Walsh等[33]两项研究也报道了相似结果。总之, 相比于18~59岁的健康人群, 老年人群按照相同的程序接种同种疫苗后, 血清中所检测到的GMT显著偏低, 但相应地老年人群中不良反应发生率也显著偏低[20, 27, 32-33]。
2.4.4. 接种程序差异的影响
虽然多项研究设计了不同接种程序的对比, 但试验结果是复杂的。Zhang等[34]的研究表明, 以2周为间隔接种疫苗的受试者获得了更快的免疫反应, 但以4周为间隔接种疫苗的受试者获得了更强的免疫反应。但Che等[28]在以2周为间隔接种疫苗的受试者中检测到了更强的免疫反应, Xia等[21]也发现以2周为间隔接种疫苗的受试者不良反应发生率低于以4周为间隔接种疫苗的受试者。在6项比较了疫苗的单剂量与双剂量接种的研究中, 4项研究显示疫苗双剂量接种比单剂量接种产生更强的免疫反应[19, 31, 33, 35]。
2.4.5. 疫苗类型差异的影响
Polack等[20]报道的RNA疫苗(BNT162b2)和Voysey等[19]报道的腺病毒重组载体疫苗(ChAdOx1 nCoV-19)受试者人数超过10 000人, 都采用相对危险度计算有效率, 显示前者有效率为95%[20], 后者有效率为70.4%[19]。其他临床试验的设计存在差异, 受试者规模较小, 结局指标也有所不同, 其有效率尚无法比较。
3. 讨论
本系统评价得出以下结论: (1)除了Zhu等[35]报道的疫苗外, 所有候选疫苗都具有良好的免疫原性和安全性。接种后28 d内, 受试者血清GMT显著增加, 血清转化率大多大于80%, 大部分疫苗的不良反应率低于30%, 且以轻到中度为主, 24 h内缓解。(2)接种后产生的效价和不良反应率与剂量呈正相关, 因此, 大部分临床试验进入Ⅲ期阶段后, 选择了中等剂量作为标准剂量, 这可能是对有效性和安全性综合考虑的结果。(3) 相同条件下, 疫苗对60岁以上的老年人的免疫原性较差, 但不良反应率也偏低, 一种可能的解释是这与人体的免疫衰老有关。老年人群对疫苗的耐受性需要继续研究。此外, 目前尚没有针对未成年人的临床试验结果发表。(4)大部分疫苗研究都推荐双剂量接种, 但接种间隔时间需进一步研究。
然而, 本系统评价有一定的局限性: (1)缺乏疫苗的长期有效性和安全性的证据。由于疫苗研发的急迫性, 大部分试验只随访到了接种后28 d, 中和性抗体能否长期维持, 接种疫苗后是否有迟发的不良反应, 仍需要更长时间的随访。(2)为了纳入更多最新证据, 本系统评价也将预印本文献包含在内, 这些文献没有经过同行评议, 且其中一些数据无法获取。(3)本系统评价只纳入了随机、双盲、对照试验, 而观察性研究、回顾性病例分析及早期的动物试验均被排除在外。如Anderson等[36]实施的一项开放标签试验发现mRNA-1273疫苗在老年人群体具有较好的安全性, Logunov等[37]在非随机临床试验中报道了两种腺病毒重组载体疫苗制剂(rAd26-S和rAd5-S)在18~60岁健康人群具有较好的安全性和免疫原性。(4)各项临床试验的设计存在差异, 导致无法对不同类型疫苗的优劣进行比较, 如Voysey等[19]和Polack等[20]采用相对危险度计算有效率, Keech等[30]未做病毒中和试验, 其余10项研究虽然均完成了病毒中和试验, 但试验设计方案差异较大[21-22, 27-29, 31-35]。(5)本系统评价只检索了中英文文献, 以日文、法文等其他语言发表的文献被排除在外。
综上所述, 本系统评价总结了COVID-19疫苗相关的临床试验结果, 表明大部分疫苗都具有较好的安全性和有效性。这让我们有理由相信, 随着COVID-19疫苗的广泛接种, 有望控制、终结COVID-19的全球大流行。
利益冲突声明:所有作者均声明不存在利益冲突。
Biographies
邢凯, 男, 本科生
Jiang Y, Email: jiangyiwd@163.com
Funding Statement
中央高校基本科研业务费专项资金资助项目(2042020kf1011)
Fundamental Research Funds for the Central Universities (2042020kf1011)
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard[EB/OL]. (2021-02-15)[2021-02-16]. https://covid19.who.int/.
- 2.Sun JM, He WT, Wang LF, et al. COVID-19:epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26(5):483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xi JP. Remarks at the National Commendation Conference on COVID-19 (8th September 2020) Qiushi. 2020;(20):4–15. [Google Scholar]; 习 近平. 在全国抗击新冠肺炎疫情表彰大会上的讲话(2020年9月8日) 求是. 2020;(20):4–15. [Google Scholar]
- 4.Zhu N, Zhang DY, Wang WL, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People's Republic of China The clinical significance of COVID-19 Diagnosis and Treatment Guidelines (Interim version 8) Journal of Tropical Diseases and Parasitology. 2020;13(5):321–328. [Google Scholar]; 中华人民共和国国家卫生健康委员会 新型冠状病毒肺炎诊疗方案(试行第八版) 中华临床感染病杂志. 2020;13(5):321–328. [Google Scholar]
- 6.Oliveira BA, Oliveira LC, Sabino EC, et al. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo. 2020;62:e44. doi: 10.1590/s1678-9946202062044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Pan LJ, Tang S, et al. Mask use during COVID-19:a risk adjusted strategy. Environ Pollut. 2020;266(Pt 1):115099. doi: 10.1016/j.envpol.2020.115099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Liu Y, Li M, et al. Mask or no mask for COVID-19:a public health and market study. PLoS One. 2020;15(8):e0237691. doi: 10.1371/journal.pone.0237691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.General Office of the National Health Commission, PRC Technical guidelines on prevention and control of novel coronavirus infection in medical institutions (First edition) Chinese Journal of Infection Control. 2020;19(2):189–191. [Google Scholar]; 中华人民共和国国家卫生健康委员会办公厅 医疗机构内新型冠状病毒感染预防与控制技术指南(第一版) 中国感染控制杂志. 2020;19(2):189–191. [Google Scholar]
- 10.Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19:an evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardo J, Shukla AM, Chamarthi G, et al. The journey of Remdesivir: from Ebola to COVID-19. Drugs Context. 2020;9:2020–2024. doi: 10.7573/dic.2020-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirupathi R, Bharathidasan K, Palabindala V, et al. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med. 2020;28(Suppl 1):57–63. [PubMed] [Google Scholar]
- 13.Provenzani A, Polidori P. COVID-19 and drug therapy, what we learned. Int J Clin Pharm. 2020;42(3):833–836. doi: 10.1007/s11096-020-01049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero JR, Bernstein HH. COVID-19 vaccines: a primer for clinicians. Pediatr Ann. 2020;49(12):e532–e536. doi: 10.3928/19382359-20201116-01. [DOI] [PubMed] [Google Scholar]
- 15.Sharma O, Sultan AA, Ding H, et al. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korang SK, Juul S, Nielsen EE, et al. Vaccines to prevent COVID-19:a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project) Syst Rev. 2020;9(1):262. doi: 10.1186/s13643-020-01516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JL, Peng Y, Xu HY, et al. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21(6):225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia SL, Duan K, Zhang YT, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu J, Yu Q, Yin ZF, et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine[J]. medRxiv, 2020. DOI: 10.1101/2020.09.27.20189548.Epubaheadofprint.
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions[M]. 2nd ed. Chichester, UK: John Wiley & Sons, 2019.
- 26.Cumpston M, Li TJ, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia SL, Zhang YT, Wang YX, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Che YC, Liu XQ, Pu Y, et al. Randomized, double-blinded, placebo-controlled phase 2 trial of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in healthy adults[J]. Clin Infect Dis, 2020. DOI: 10.1093/cid/ciaa1703.Epubaheadofprint.
- 29.Ella R, Vadrevu KM, Jogdand H, et al. A phase 1: safety and immunogenicity trial of an inactivated SARS-CoV-2 vaccine-BBV152[J]. medRxiv, 2020. DOI: 10.1101/2020.12.11.20210419.Epubaheadofprint.
- 30.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/Ⅱ study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 32.Richmond P, Hatchuel L, Dong M, et al. A first-in-human evaluation of the safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit vaccine for COVID-19 in healthy adults; a phase 1, randomised, double-blind, placebo-controlled trial[J]. medRxiv, 2020. DOI: 10.1101/2020.12.03.20243709.Epubaheadofprint.
- 33.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YJ, Zeng G, Pan HX, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


