Abstract
Objective:
To assess the association of conventional semen parameters and sperm DNA fragmentation with risk of recurrent spontaneous abortion (RSA).
Design:
Systematic review and meta-analysis.
Setting:
Not applicable.
Patient(s):
Total 1,690 male partners of women with RSA, and 1,337 male partners of fertile control women.
Intervention(s):
Case-control or cohort studies were determined by searching PubMed, Google Scholar, Cochrane Libraries, China Biology Medicine disc, Chinese Scientific Journals Fulltext Database, China National Knowledge Infrastructure, and Wanfang Database. RSA was defined as two or more previous pregnancy losses. The fertile women refer to the reproductive women who have had at least a normal pregnancy history and no history of abortion.
Main Outcome Measure(s):
This study included eight outcome measures: semen volume(ml), semen pH value, sperm density(106/ml), sperm viability (%), sperm progressive motility rate (%), normal sperm morphology rate (%), sperm deformity rate(%), sperm DNA fragmentation index (DFI) (%). The summary measures were reported as standardized mean difference (SMD) with 95% confidence interval (CI).
Result(s):
Finally, twenty-four studies were included for analysis. Overall, male partners of women with RSA had a significantly lower level of sperm density (SMD = -0.53, 95%CI: – 0.75 to –0.30), sperm viability (SMD = -1.03, 95%CI: – 1.52 to –0.54), sperm progressive motility rate (SMD = -0.76, 95%CI:-1.06 – -0.46), and normal sperm morphology rate (SMD = –0.56, 95%CI: – 0.99 to –0.12), and had a significantly higher rate of sperm deformity rate (SMD = 1.29, 95%CI: 0.60 – 1.97), and sperm DFI (SMD = 1.60, 95%CI: 1.04 to 2.17), when compared with the reference group. However, there were no statistically significant differences for semen volume (SMD = -0.03, 95%CI: -0.14 – 0.08) and semen pH value (SMD = –0.23, 95% CI: –0.50 to 0.05) among 2 groups.
Conclusion(s):
The results of this analysis support an association of sperm density, sperm viability, sperm progressive motility rate, normal sperm morphology rate, sperm deformity rate, as well as sperm DFI with RSA. However, given the significant heterogeneity between studies and the lack of more detailed data on the subjects, further large-scale prospective studies are needed.
Keywords: Chinese population, conventional semen parameters, meta-analysis, recurrent spontaneous abortion, sperm DNA fragmentation
1. Introduction
According to guidelines of the World Health Organization (WHO), miscarriage refers to the loss of a fetus or embryo before 20 to 22 weeks of pregnancy, when the fetal weight does not exceed 500 grams.[1] Recurrent spontaneous abortion (RSA), as a devastating reproductive problem faced by couples tying to expand their families, is usually defined as three or more consecutive miscarriages with the same spouse before 28 weeks of pregnancy.[2] However, The definition of RSA differs among international societies. According to the American Society for Reproductive Medicine,[1] it is defined as two or more clinical pregnancy losses, but not necessarily consecutive. In view of the great increase in the possibility of repeated abortion after two consecutive spontaneous abortions, many Chinese scholars have included two consecutive spontaneous abortions in the scope of RSA in recent years. The incidence of RSA among couples of childbearing age was about 1% to 5%, which is not high, but has a great impact on the family happiness and psychological state of the patients.[3]
In the past few decades, there has been a rapidly growing interest in exploring the pathogenesis of RSA. Although many epidemiological studies[4,5] have attempted to test this topic and identified some risk factors, such as reproductive tract infections, gene mutations, bad eating habits or lifestyles, uterine anatomical abnormalities, endocrine disorders, and immune factors, there were approximately 40% to 50% of patients who were left without an answer.[6] Undoubtedly, this is a huge challenge for doctors and patients seeking the causes and effective treatment of RSA. Therefore, it is critically important to explore additional reasons of RSA. Previous studies mainly focused on the influence of maternal factors on RSA. However, since the genes of human embryos and their products come from both males and females, part of the causes of RSA may come from males, including genetic factors, sperm quantity and quality. Human sperm are affected by environmental factors (including oxidative stress, chemical and toxicants, radiation and trace element deficiency, etc) during the maturation process, causing sperm chromosome karyotype, DNA and genetic mutations, leading to abnormal sperm morphology and function. In recent years, a large number of studies have shown that male sperm quality was significantly associated with infertility, pregnancy rate and embryo quality.[7–9] Clinically, male sperm quality is usually measured by semen volume, semen pH value, sperm density, sperm viability, sperm progressive motility rate, normal sperm morphology rate, sperm deformity rate, and sperm DNA fragmentation index (DFI). Some studies have investigated the link between male sperm quality and risk of RSA,[10–13] but the magnitudes of the association varied between studies and even conflicting results were found, which may be partly due to insufficient sample size, resulting in low statistical efficiency and unstable results.
So far, four meta-analyses[14–17] have been conducted on this topic. However, these reviews only focused on the association of 1 or 2 parameters (i.e., sperm DFI and sperm morphology) of sperm with risk of RSA, while the association of other conventional semen parameters, including volume, pH value, density, viability and progressive motility rate with risk of RSA are hardly assessed. In fact, in China, there were many original studies that tried to address this issue. Nevertheless, most of Chinese studies have not been included by previous reviews[14–17] because of published language. Furthermore, there is a lack of meta-analysis for the Chinese population on this topic. Given the inconsistency of the existing literatures and the insufficient statistical power of primary studies, therefore, we conducted a meta-analysis to examine whether the conventional semen parameters were significantly associated with occurrence of RSA.
2. Materials and methods
This study was approved by the ethics committee of Xiangya School of Public Health, Central South University.
2.1. Literature search
We tried to report this meta-analysis following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.[18] PubMed, Google Scholar, Cochrane Libraries, China Biology Medicine disc, Chinese Scientific Journals Fulltext Database (CQVIP), China National Knowledge Infrastructure, and Wanfang Database were searched, with an end date parameter of January 2019, to identify the relevant studies that assessed the association between conventional semen parameters and RSA. The following search terms were used:
-
(1)
recurrent spontaneous abortion/RSA, repetitive spontaneous abortion, habitual spontaneous abortion, recurrent pregnancy loss, repetitive pregnancy loss/RPL, habitual pregnancy loss, repetitive miscarriage, recurrent miscarriage, habitual miscarriage, habitual misbirth, recurrent misbirth, repetitive misbirth, pregnancy loss, abortion, and miscarriage;
-
(2)
sperm, semen, sperm quality, DNA fragmentation, semen volume, semen pH, sperm density, sperm viability, sperm progressive motility, sperm morphology, sperm deformity, and semen parameters; and
-
(3)
prospective studies, follow-up studies, cohort studies, longitudinal studies, and case-control studies.
-
(4)
Reference lists of the retrieved papers were also reviewed. Furthermore, we contacted authors of the primary studies for additional information.
2.2. Definitions and selection criterions
In the present review, the indicators of interest were conventional semen parameters including semen volume (ml), semen pH value, sperm density (106/ml), sperm viability (%), sperm progressive motility rate (%), normal sperm morphology rate (%), sperm deformity rate (%), and sperm DFI (%). The outcomes of interest were RSA which was defined as two or more previous pregnancy losses. Male partners of women with RSA were defined as the case group; and male partners of fertile control women were defined as the control group. Studies were considered to be eligible if they met the following criterions:
-
(1)
the study design was a case-control or cohort study;
-
(2)
definition of RSA was clear;
-
(3)
study participants were Chinese populations;
-
(4)
studies compared the differences of conventional semen parameters between male partners of women RSA and male partners of fertile women;
-
(5)
the exposures of interest were conventional semen parameters;
-
(6)
the outcomes of interest were RSA;
-
(7)
the study was published in Chinese or English language; and
-
(8)
corresponding data were provided.
-
(9)
Review articles, conference abstracts and presentations were excluded. Additionally, if two or more studies used the same data, we only included the study with the highest quality.
2.3. Data extraction and quality assessment
A standardized data collection form was used to extract data. We extracted any reported mean and standard deviation of every conventional semen parameter for every group. Additionally, the characteristics of each study were extracted. Information was recorded as follows: first author; publication year; study period; geographic region; Study design; sample size of case and control group; reported semen parameters; whether to control confounding factors and quality scores.
We used the Newcastle-Ottawa Scale (available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) to assess the risk of bias in the included studies. Using the tool, each study was judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the ascertainment of outcome or exposure. Stars awarded for each quality item serve as a quick visual assessment. Stars are awarded such that the highest quality studies are awarded up to nine stars. When the study gains at least seven stars, it is considered of low-risk of bias. Two independent reviewers (LJQ and QJB) selected literatures, extracted data and assessed study quality. Any disagreements were resolved through discussion among the authors until consensus was reached.
2.4. Statistical analysis
Considering this fact that conventional semen parameters belonged to quantitative data, we used standardized mean difference (SMD) to assess the association between these parameters and risk of RSA. Homogeneity of effect size across studies was tested by using the Q statistics at the P < .10 level of significance. The I2 statistic, as a quantitative measure of inconsistency across studies, was also calculated (significance level at I2 > 50%). SMDs and their corresponding 95% confidence intervals (CI) were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models. Because quality scores and adjustments for confounding factors were inconsistent across studies, we further conducted a sensitivity analysis to explore possible explanations for heterogeneity and to examine the influence of various exclusion criteria on the overall combined SMD. We also conducted a sensitivity analysis to investigate the influence of a single study on the overall risk estimate by omitting one study at a time. Potential publication bias was assessed by Begg funnel plots and Egger linear regression test. Statistical tests were declared significant for a 2-sided P value not exceeding .05, except where otherwise specified. Statistical analyses were performed by using Review Manager version 5.0 (The Nordic Cochrane Centre, The Cochrane Collaboration) and Stata version 12.0 (StataCorp LP, College Station, TX).
3. Results
3.1. Literature search
After the computerized search in seven databases, we initially identified 864 potentially eligible records. Of these, the majority were excluded after the first screening based on abstracts or titles, mainly because they were duplicates, reviews, conference papers, or not relevant to our analysis. After full-text review of 109 studies, 24 case-control studies [12–13,19–40] met the inclusion criteria (Fig. 1). Reasons for not including the other studies were:
Figure 1.
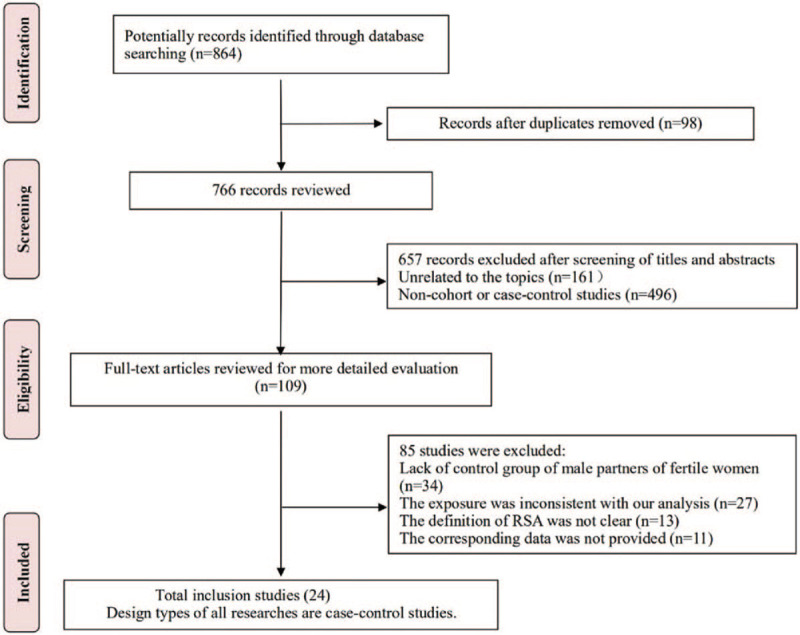
Flow chart of study identification and selection.
-
(1)
lack of control group of male partners of fertile women (n = 34);
-
(2)
the exposure was inconsistent with our analysis (n = 27);
-
(3)
the definition of RSA was not clear (n = 13); and
-
(4)
the corresponding data was not provided (n = 11).
3.2. Study characteristics
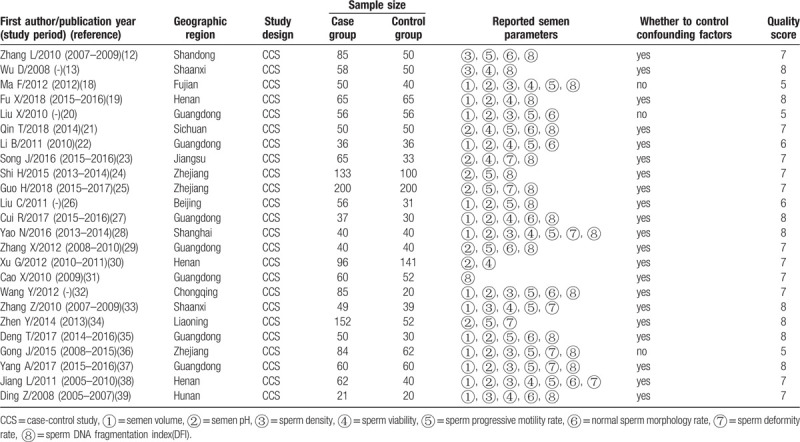
The characteristics of included studies, which involved 1690 male partners of women with RSA and 1,337 corresponding controls, and were published between 2008 and 2018, are summarized in Table 1. All included studies belonged to case-control studies and provided a clear definition of RSA. In all included studies, patients with RSA did not have clear cause or genital dysplasia, infection and other serious primary diseases; male partners did not have serious physical, infectious or psychosis diseases, infertility history in the family, urinary tract infection and tumor history, genital tract defect, deformity and corresponding surgical history, and severe sperm diseases. Only three studies (12.5%) did not control any confounding factor when assessing the association of conventional semen parameters with RSA. The quality scores in most included studies (79.2%) were more than seven scores. Among the 24 studies included here, the number of studies reporting conventional semen parameters was as follows: 14 semen volume; 8 semen pH value; 23 sperm density; 13 sperm viability; 17 sperm motility rate; 10 normal sperm morphology rate; 8 sperm deformity rate; and 18 sperm DFI.
Table 1.
Characteristics of 24 case-control studies assessing the association between male sperm quality and risk of RSA.
3.3. Male semen parameters and RSA
Overall, there were no statistically significant differences for semen volume (SMD = -0.03, 95%CI: -0.14 to 0.08; P = .55) and pH value (SMD = -0.23, 95%CI: -0.50 to 0.05; P = .11) between the male partners of women with RSA and the corresponding controls (Fig. 2).
Figure 2.
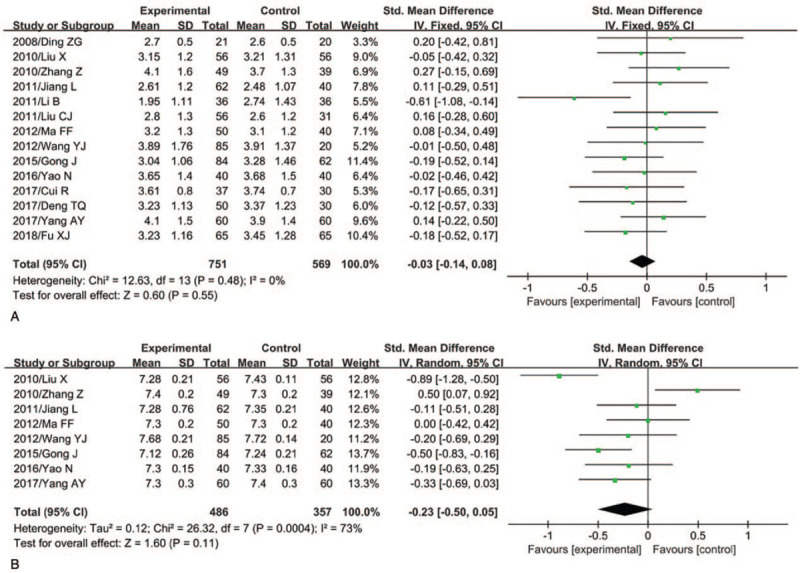
Semen volume and pH value and risk of RSA (A, semen volume; B, pH value).
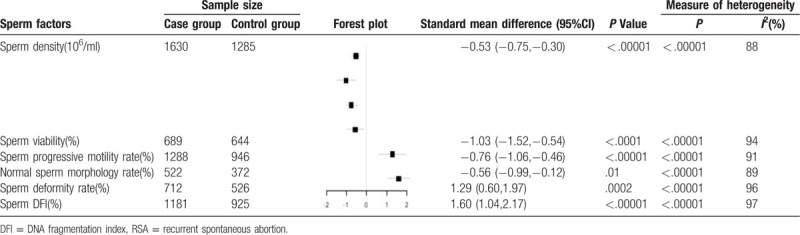
However, the male partners of women with RSA were at a significantly lower level for sperm density (SMD = -0.53, 95%CI:-0.75 to -0.30; P < .00001), sperm viability (SMD = -1.03, 95%CI:-1.52 to -0.54); P < .0001), sperm progressive motility rate (SMD = -0.76, 95%CI:-1.06 to -0.46; P < .00001), and normal sperm morphology rate (SMD = -0.56, 95%CI:-0.99 to -0.12; P = .01), and were at a significantly higher level for sperm deformity rate (SMD = 1.29, 95%CI: 0.60 to 1.97; P = .0002) and sperm DFI (SMD = 1.60, 95%CI: 1.04 to 2.17; P < .00001), when compared with the partners of fertile control women (Table 2). Substantial heterogeneity was observed for most semen parameters except for semen volume (P = .48; I2 = 0%).
Table 2.
Remaining semen parameters associated with risk of RSA.
3.4. Sensitivity analyses
Sensitivity analyses were conducted to explore potential sources of heterogeneity in the association between conventional semen parameters and RSA to examine the influence of various exclusion criteria on the overall risk estimate. Exclusion of five studies[19,21,23,27,37] that belonged to low-quality studies with a score less than 7, yielded similar results for all conventional semen parameters including semen volume (SMD = 0.02, 95%CI: -0.13 to 0.16, P = .82), semen pH value (SMD = -0.07, 95% CI: -0.36 to 0.21, P = .62), sperm density (SMD = -0.59, 95%CI:-0.87 to -0.32, P < .0001), sperm viability (SMD = -1.15, 95%CI:-1.73 to -0.58), P < .0001), sperm progressive motility rate (SMD = -0.76, 95%CI:-1.08 to -0.44, P < .00001), and normal sperm morphology rate (SMD = -0.64, 95%CI:-1.16 to -0.12, P = .02), sperm deformity rate (SMD = 1.24, 95%CI: 0.46 to 2.03, P = .002), and sperm DFI (SMD = 1.99, 95%CI: 1.47 to 2.51, P < .00001). Exclusion of 3 studies [19,21,37] in which any confounding factor did not be controlled when assessing the association between semen parameters and RSA, did not change the overall risk estimate including semen volume (SMD = -0.02, 95%CI: -0.15 to 0.11, P = .78), semen pH value (SMD = -0.07, 95% CI: -0.36 to 0.21, P = .62), sperm density (SMD = -0.57, 95%CI:-0.82 to -0.32, P < .00001), sperm viability (SMD = -1.07, 95%CI:-1.61 to -0.54, P < .0001), sperm progressive motility rate (SMD = -0.68, 95%CI:-0.97 to -0.39, P < .00001), and normal sperm morphology rate (SMD = -0.54, 95%CI:-1.02 to -0.06, P = .03), sperm deformity rate (SMD = 1.24, 95%CI:0.46 to 2.03, P = .002), and sperm DFI (SMD = 1.89, 95%CI: 1.39 to 2.39, P < .00001). Further exclusion of any single study did not materially alter the overall combined SMD (Figs. 3 and 4).
Figure 3.
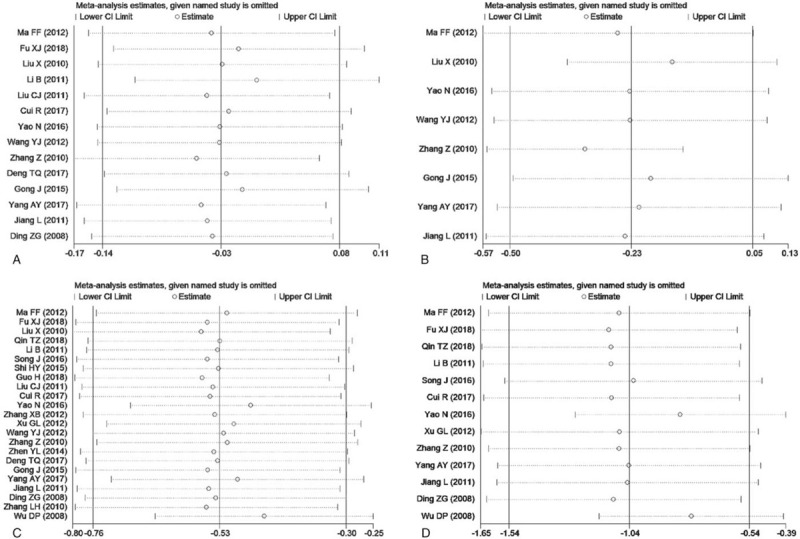
Sensitivity analysis chart for semen volume, pH value, density and viability (A, semen volume; B, semen pH value; C, sperm density; D, sperm viability).
Figure 4.
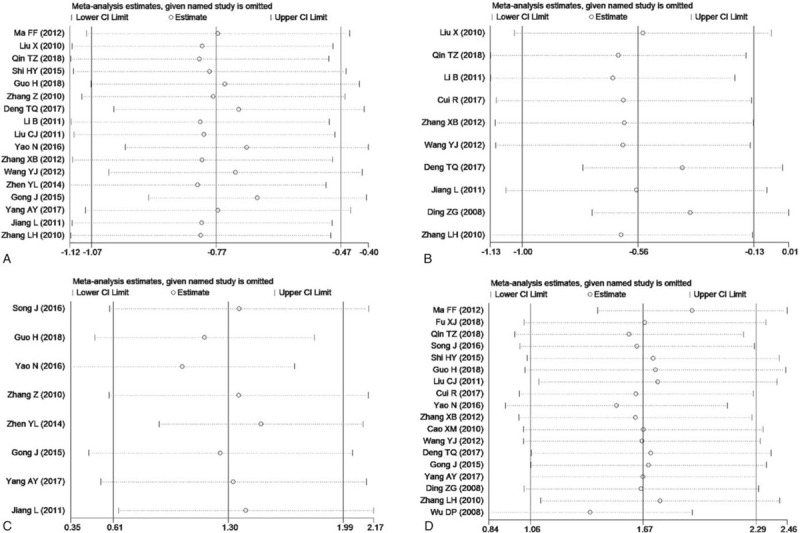
Sensitivity analysis chart for the remaining four indicators. (A, sperm progressive motility rate; B, normal sperm morphology rate; C, sperm deformity rate; D, sperm DFI).
3.5. Publication bias
The Begg's funnel plot did not show any substantial asymmetry except for sperm viability and sperm deformity rate (Fig. 5). However, Egger's regression test indicated that there was no the evidence of publication bias (P = .869 for semen volume; 0.364 for semen pH value; 0.051 for sperm density; 0.464 for sperm viability; 0.530 for sperm progressive motility rate; 0.851 for normal sperm morphology rate; 0.386 for sperm deformity rate; and 0.938 for sperm DFI).
Figure 5.
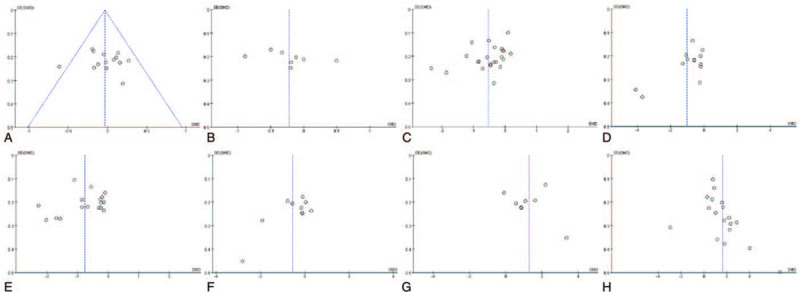
Funnel chart (A, semen volume; B, semen pH value; C, sperm density; D, sperm viability; E, sperm progressive motility rate; F, normal sperm morphology rate; G, sperm deformity rate; H, sperm DFI).
4. Discussion
In the past few decades, there has been a rapidly growing interest in the etiology of RSA and its intervention targets, but so far, there is still no clear clue to these problems. Clinically, male semen parameters are often used in the diagnosis and treatment targets of sub-fertility, which indicates male sperm quality may be associated with the occurrence of RSA. The present meta-analysis of 24 case-control studies, which included 1690 male partners of women with RSA and 1,337 corresponding controls, with sufficient statistical power, aimed to assess the association of male semen parameters (e.g., semen volume, semen pH value, sperm density, sperm viability, sperm progressive motility rate, normal sperm morphology rate, sperm deformity rate and sperm DFI) with risk of RSA.
Findings from our study showed that the male partners of RSA patients compared with the partners of fertile control women, had a significantly lower level of sperm density (SMD = -0.53), sperm viability (SMD = -1.03), sperm progressive motility rate (SMD = -0.76), and normal sperm morphology rate (SMD = -0.56), but had a significantly higher level for sperm deformity rate (SMD = 1.29) and sperm DFI (SMD = 1.60), which indicates male sperm quality was significantly associated with risk of RSA. Although we only focused on the Chinese population, as far as we know, this is the first meta-analysis to comprehensively evaluate the relationship between male sperm parameters and recurrent abortion. An improved understanding of this issue may have important clinical implications, given the possibility that the clear results might help to provide counselling and treatment targets to RSA patients.
Although many epidemiology studies have been performed to assess the association between male factors and risk of RSA, the magnitudes of the association between male sperm parameters and RSA varied across studies and even there were conflicting results. As early as 1994, Hill et al[10] began to pay attention to this issue, but they did not observe statistically significant associations between male sperm parameters such as semen volume, sperm density, normal sperm morphology rate and sperm deformity rate and RSA, which may be due to a small sample size (98 cases vs 17 controls), so it is difficult to find significant statistical differences. On the contrary, Buckett et al[11] found a statistically significant difference for sperm progressive motility rate and normal sperm morphology rate between male partners of women with RSA and fertile control women, which indicates male sperm factors may be associated with risk of RSA. In China, since 2008, many researchers have begun to pay attentions to the association between male sperm parameters and risk of RSA. For example, 14 studies reported the association of semen volume with RSA, 8 studies reported semen pH value, 23 studies reported sperm density, 13 studies reported sperm viability, 17 studies reported sperm motility rate, 10 studies reported normal sperm morphology rate, 8 studies reported sperm deformity rate, and 18 studies reported sperm DFI. Of note, in these studies from China, the sample size is generally relatively small, the magnitudes of the association varied and even there were conflicting results. In addition, in other countries, few researchers comprehensively evaluate the relationship between male sperm parameters and RSA. Therefore, it is necessary to sum up the evidence of China, which can help to provide reference for other regions and cultures.
So far, four meta-analyses[14–17] have been performed to evaluate the association between male sperm factors and RSA. However, these reviews focused on the association of sperm DNA fragmentation and sperm morphology with risk of RSA, and hardly assessed the association of other semen parameters (e.g., volume, pH value, density, viability and progressive motility rate) with risk of RSA. For example, in 2019, a review[15] of 13 prospective studies, which involved 579 male partners of women with recurrent pregnancy loss and 434 male partners fertile control women and to our knowledge is the latest meta-analysis around this topic to date, suggested that male partners of women with a history of RSA have a significantly higher rate of sperm DNA fragmentation compared to the partners of fertile control women (mean difference [MD] = 11.91; 95%CI: 4.97–18.86), which was supported by other two reviews [14,16]. Besides, Cao et al[17] conducted a systematic review and meta-analysis and found that the low level of normal morphology rate was significantly associated with RSA risk. Our findings are generally consistent with previous reviews. For example, in the present review, we found that the male partners of RSA patients were at a significantly higher level of sperm DFI (SMD = 1.60; 95%CI: 1.04–2.17), when compared with the partners of fertile control women. Additionally, among 18 included studies that assessed the relationship between sperm DFI and RSA, most of studies showed a significantly positive association. However, our study has important strengths compared with previous studies. This review is the most up to date on this subject. With the accumulating evidence and enlarged sample size, we have enhanced statistical power to provide more precise and reliable risk estimates. In our study, most included studies (79.2%) were considered of higher methodologic quality; and these high-quality studies contributed nearly 95% of study population. Furthermore, the association between male sperm parameters and risk of RSA persists and remains statistically significant in sensitivity analysis based on various exclusion criteria. Additionally, our study indicated other semen parameters including sperm density, sperm viability and sperm progressive motility rate were significantly associated with the occurrence of RSA, which has not been confirmed by previous meta-analyses[14–17].
Recently, a large sample study showed that sperm DNA fragmentation rate were significantly correlated with other routine semen parameters such as progressive motility rate (r = − 0.47, P < .001) and total motile sperm count (r = −0.31, P < .001) in Chinese couples.[41] At present, the main clinical means to reflect male reproductive capacity are still routine sperm parameter detection, including semen volume, density, vitality and morphology, and sperm DNA fragmentation rate is rarely tested. However, this analysis is based on visual estimation of sperm count, viability and morphology by optical microscopy and is difficult to perform reliably. Therefore, it is recommended to use sperm DNA integrity testing as a promising detection method for standard semen analysis.
The underlying mechanisms of the association between male sperm quality and risk of RSA remain uncertain, and few studies have explored this issue. One possible explanation is that reactive oxygen species (ROS) and aneuploidy of human sperm chromosome play a key role in the association of poor sperm quality and risk of RSA. Sperm damage is usually the result of multiple factors, among which ROS is one of known causes of sperm fragmentation and decreased sperm motility.[42,43] For example, some studies have shown that ROS can cause sperm DNA damage, leading to a range of adverse consequences, including lowering fertilization rates, disrupting pre-implantation embryo development and increasing abortion rates.[44] It was also reported that the levels of ROS in the spouses of RSA patients who had excluded female factors were 9 times higher than in the control population.[45]
Additionally, it has been reported that aneuploidy of human sperm chromosome was an important cause of poor sperm quality.[40,46] For example, some studies suggested that there were significantly positive association between total aneuploidy rate of sperm chromosomes and DFI.[47,48] When compared with male partners of fertile control women, male partners of women with RSA had a significantly increased rate of sperm aneuploidy.[49] Findings from previous studies also showed that sperm chromosome aneuploidy was an important cause of human infertility, spontaneous abortion, abnormal embryo development and birth defects.[50,51]
Potential limitations of this study should be considered. First, substantial heterogeneity was observed among studies assessing the association male sperm quality and risk of RSA, which was not surprising given the differences in characteristics of populations, ascertainment of RSA, sperm parameter detections, and adjustment for confounding factors. In our review, sensitivity analyses were used to explore potential sources of heterogeneity in the association between conventional semen parameters and RSA to examine the influence of various exclusion criteria on the overall risk estimate. As a result, sensitivity analyses yielded similar results, which indicated that our results are stable and reliable. Second, the semen status used in these studies may vary, and there may be differences in the time from the acquisition to the analysis interval. Third, the sample size was less than 150 in many included studies, so more studies should be included in future reviews, to provide further support for our results. Fourth, residual confounding is of concern. Uncontrolled or unmeasured risk factors potentially produce biases. Although only three studies (12.5%) among all included studies did not control any confounding factor when assessing the association of conventional semen parameters with RSA, in most of the remaining studies only several common confounders were considered. Besides, potential publication bias could influence the findings. In the present study, the Begg's funnel plot did show some substantial asymmetry for two semen parameters including sperm viability and sperm deformity rate. However, Egger's regression test indicated that there was no the evidence of publication bias. Last but not least, because the present review only included studies in which study participants were Chinese populations, additional research in other populations is warranted to generalize the findings.
In conclusion, the present study aimed at addressing the association of male semen parameters with risk of RSA. Although the role of potential bias and evidence of heterogeneity should be carefully evaluated, our study indicated that the levels of routine semen parameters including sperm density, sperm viability, sperm progressive motility rate, normal sperm morphology rate, sperm deformity rate, as well as sperm DFI were significantly different between male partners of women with RSA and those of fertile control women. However, given the significant heterogeneity between studies and the lack of more detailed data on the subjects, further large-scale prospective studies are needed.
Author contributions
Conceptualization: Jinqi Li.
Data curation: Jinqi Li, Liu Luo, Jingyi Diao.
Formal analysis: Jinqi Li.
Funding acquisition: Jiabi Qin.
Investigation: Jinqi Li, Liu Luo, Jingyi Diao, Yihuan Li.
Methodology: Jinqi Li, Jingyi Diao.
Project administration: Senmao Zhang, Letao Chen, Tubao Yang, Jiabi Qin.
Resources: Tubao Yang, Jiabi Qin.
Software: Liu Luo, Jingyi Diao, Yihuan Li.
Supervision: Jiabi Qin.
Validation: Liu Luo, Yihuan Li.
Visualization: Senmao Zhang, Letao Chen.
Writing – original draft: Jinqi Li.
Writing – review & editing: Jiabi Qin.
Footnotes
Abbreviations: CI = confidence intervals, DFI = DNA fragmentation index, ROS = reactive oxygen species, RSA = recurrent spontaneous abortion, SMD = standardized mean difference.
How to cite this article: Li J, Luo L, Diao J, Li Y, Zhang S, Chen L, Yang T, Qin J. Male sperm quality and risk of recurrent spontaneous abortion in Chinese couples: a systematic review and meta-analysis. Medicine. 2021;100:10(e24828).
This study was supported by the Project Funded by National Natural Science Foundation Program of China (82073653, 81803313 and 81974019), Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), Natural Science Foundation of Hunan Province (2018JJ2551), Hunan Provincial Key Research and Development Program (2018SK2063 and 2018SK2062), Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006), and China Postdoctoral Science Foundation (2020M682644).
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (JB Qin) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Evaluation and treatment of recurrent pregnancy loss: a committee, opinion. Fertil Steril 2012;98:1103–11. [DOI] [PubMed] [Google Scholar]
- [2].Kolte AM, Bernardi LA, Christiansen OB, et al. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod 2015;30:495–8. [DOI] [PubMed] [Google Scholar]
- [3].Huang M. Etiological analysis and treatment of recurrent spontaneous abortion. Journal of Practical Gynecologic Endocrinology 2017;4:101–2. [Google Scholar]
- [4].Imam SN, Shamsi MB, Kumar K, et al. Idiopathic recurrent pregnancy loss: role of paternal factors; a pilot study. J Reprod Infertil 2011;12:267–76. [PMC free article] [PubMed] [Google Scholar]
- [5].Bellver J, Meseguer M, Muriel L, et al. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod 2010;25:1713–21. [DOI] [PubMed] [Google Scholar]
- [6].Porter TF, Scott JR. Evidence-based care of recurrent miscarriage. Best Pract Res Clin Obstet Gynaecol 2005;19:85–101. [DOI] [PubMed] [Google Scholar]
- [7].Hamamah S, Fignon A, Lansac J. The effect of male factors in repeated spontaneous abortion: lesson from in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod Update 1997;3:393–400.9459284 [Google Scholar]
- [8].Gil-Villa AM, Cardona-Maya W, Agarwal A, et al. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril 2010;94:1465–72. [DOI] [PubMed] [Google Scholar]
- [9].Kamkar N, Ramezanali F, Sabbaghian M. The relationship between sperm DNA fragmentation, free radicals and antioxidant capacity with idiopathic repeated pregnancy loss. Reprod Biol 2018;18:330–5. [DOI] [PubMed] [Google Scholar]
- [10].Hill JA, Abbott AF, Politch JA. Sperm morphology and recurrent abortion∗∗Supported by grants HD00815 and HD23547 from the National Institutes of Health, Bethesda, Maryland, and the Fearing Research Laboratory Endowment, Brigham and Women's Hospital, Harvard Medical School, Boston, Mass. Fert Ster 1994;61:776–8. [PubMed] [Google Scholar]
- [11].Buckett WM, Luckas MJ, Aird IA, et al. The hypo-osmotic swelling test in recurrent miscarriage. Fertil Steril 1997;68:506–9. [DOI] [PubMed] [Google Scholar]
- [12].Zhang L, Jiang P, Liu Y, et al. Relationship between sperm DNA integrity and the prognosis of pregnancy for patients with recurrent spontaneous abortion. Progress in Obstetrics & Gynecology 2010;19:673–5. [Google Scholar]
- [13].Wu D, Wu Q, Li X. Relationship between recurrent spontaneous abortions and sperm DNA integrity. Journal of Modern Urology 2008;13:132–4. [Google Scholar]
- [14].Tan J, Taskin O, Albert A, et al. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online 2019;38:951–60. [DOI] [PubMed] [Google Scholar]
- [15].McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril 2019;112:54–60.e3. [DOI] [PubMed] [Google Scholar]
- [16].Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908–17. [DOI] [PubMed] [Google Scholar]
- [17].Cao X, Cui Y, Zhang X, et al. The correlation of sperm morphology with unexplained recurrent spontaneous abortion: a systematic review and meta-analysis. Oncotarget 2017;8:55646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [19].Ma F, Wang H, Zhou Y, et al. Relationship between the prognosis of pregnancy for patients with recurrent spontaneous abortion and sperm DNA integrity. Chinese Journal of Birth Health & Heredity 2012;20:64–5. [Google Scholar]
- [20].Fu X, Men B, Sun Z, et al. Study on the relationship between the integrity of sperm DNA and unexplained recurrent spontaneous abortion. Chinese Journal of Human Sexuality 2018;27:132–4. [Google Scholar]
- [21].Liu X, Chen Z. The relationship between the Semen Analysis and Sperm Morphology Analysis and Habitual Abortion. China Practical Medicine 2010;5:18–9. [Google Scholar]
- [22].Qin T, Xu K, Liu L, et al. Study of comet assay for detecting sperm DNA integrity of patients with unexplained recurrent spontaneous abortion. Chinese Journal of Family Planning 2018;26:260–5+269. [Google Scholar]
- [23].Li B, Zhou Q, Zhu Z, et al. Recurrent miscarriage and the quality of semen and sperm: A case-control study. National Journal of Andrology 2011;17:596–600. [PubMed] [Google Scholar]
- [24].Song J, Wang Q, Sun H, et al. Relationship between sperm DNA fragmentation index and recurrent spontaneous abortion and its correlation with semen parameters. Journal of Nantong University (Medical Sciences) 2016;36:571–3. [Google Scholar]
- [25].Shi H, Zheng J, Zhou L, et al. Effect of Sperm DNA fragmentation on male infertility and early recurrent miscarriage. China Modern Doctor 2015;53:72–5. [Google Scholar]
- [26].Guo H, Jin M, Jin D, et al. Relationship between sperm DNA fragmentation and recurrent spontaneous abortion and sperm parameters. Clinical Education of General Practice 2018;16:134–6. [Google Scholar]
- [27].Liu C, Wang A, Shang W, et al. Sperm DNA damage and unexplained recurrent spontaneous abortion. National Journal of Androlog 2011;17:619–21. [PubMed] [Google Scholar]
- [28].Cui R, Zhong X, Miao Z, et al. Effects of sperm DNA integrity on recurrent spontaneous abortions. Hainan Medical Journal 2017;28:2034–6. [Google Scholar]
- [29].Yao N, Xu Y, He Y. Correlation of sperm chromatin structure and unexplained recurrent miscarriage. Chinese Journal of Human Sexuality 2016;25:102–4. [Google Scholar]
- [30].Zhang X, Wei J, Fang X, et al. Clinical Value of Sperm Chromatin Diffusion Test in Early Unexplained Recurrent Abortion. Chinese Journal of Coal Industry Medicine 2012;15:822–3. [Google Scholar]
- [31].Xu G, Zhang F, Sun H. Relationship between abnormal sperm morphology and recurrent spontaneous abortion. China Medical Engineering 2012;20:44–5. [Google Scholar]
- [32].Cao X, Su Y, He Q, et al. Effect of sperm factors on unexplained recurrent spontaneous abortion. The Journal of Practical Medicine 2010;26:3142–4. [Google Scholar]
- [33].Wang Y, Li D, Zhang W, et al. Correlation of recurrent pregnancy loss with sperm parameters and sperm DNA fragmentation. Chinese Journal of Medical Genetics 2012;29:602–5. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Z, Shi J, Xing J, et al. Correlation of recurrent miscarriage with semen parameters, teratozoo-spermia and DNA integrity. Journal of Third Military Medical University 2010;32:1788–92. [Google Scholar]
- [35].Zhen Y. The analysis of sperm DNA integrity and unexplained recurrent miscarriage. China: Dalian Medical University; 2014. [Google Scholar]
- [36].Deng T, Deng M, Wang Y, et al. Correlation between sperm nuclear maturity and earlyunexplained recurrent miscarriage. Chinese Journal of Human Sexuality 2017;26:116–8. [Google Scholar]
- [37].Gong J, Lou W, Li Q. Analysis of the man factors on recurrent abortion. Chinese Journal of Health Laboratory Technology 2015;25:2960–2. [Google Scholar]
- [38].Yang A, Qin J. Study on the correlation of recurrent spontaneous abortion with semen parameters, sperm deformity rate and DNA integrity. Clinical Medicine 2017;37:37–8. [Google Scholar]
- [39].Jiang L. Recurrent pregnancy loss and sperm quality and form abnormal discuss the relation. China: Zhengzhou University; 2011. [Google Scholar]
- [40].Ding Z, Nie H, Zhu W, et al. Effect of sperm factor on recurrent pregnancy loss. Journal of Chinese Physician 2008;10:1045–7. [Google Scholar]
- [41].Zhu XB, Chen Q, Fan WM, et al. Sperm DNA fragmentation in Chinese couples with unexplained recurrent pregnancy loss. Asian J Androl 2019;22:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaare M, Painter JN, Ulander VM, et al. Sex chromosome characteristics and recurrent miscarriage. Fertil Steril 2008;9:2328–33. [DOI] [PubMed] [Google Scholar]
- [43].Dewan S, Puscheck EE, Coulam CB, et al. Y-chromosome microdeletions and recurrent pregnancy loss. Fertil Steril 2006;85:441–5. [DOI] [PubMed] [Google Scholar]
- [44].Deng L, Lu T, Chen J, et al. Research progress on the effect of reactive oxygen species on sperm function. Chinese Journal of Andrology 2016;30:61–3+72. [Google Scholar]
- [45].Shamsi MB, Venkatesh S, Pathak D, et al. Sperm DNA damage & oxidative stress in recurrent spontaneous abortion (RSA). Indian J Med Res 2011;133:550–1. [PMC free article] [PubMed] [Google Scholar]
- [46].Carrell DT, Wilcox AL, Lowy L, et al. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol 2003;101:1229–35. [DOI] [PubMed] [Google Scholar]
- [47].Tang SS, Gao H, Zhao Y, et al. Aneuploidy and DNA fragmentation in morphologically abnormal sperm. Int J Androl 2010;33:e163–79. [DOI] [PubMed] [Google Scholar]
- [48].Brahem S, Elghezal H, Ghedir H, et al. Cytogenetic and molecular aspects of absolute teratozoospermia: comparison between polymorphic and monomorphic forms. Urology 2011;78:1313–9. [DOI] [PubMed] [Google Scholar]
- [49].Rubio C, Simon C, Blanco J, et al. Implications of sperm chromosome abnormalities in recurrent miscarriage. J Assist Reprod Genet 1999;16:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Martin RH. Mechanisms of nondisjunction in human spermatogenesis. Cytogenet Genome Res 2005;111:245–9. [DOI] [PubMed] [Google Scholar]
- [51].Peng X, Huang C, Fan L, et al. Research progress on aneuploid of human sperm chromosome. Journal of Reproductive Medicine 2017;26:1109–13. [Google Scholar]


