Figure 3. Structures of bacterial SQOR, FCSD, and human SQOR.
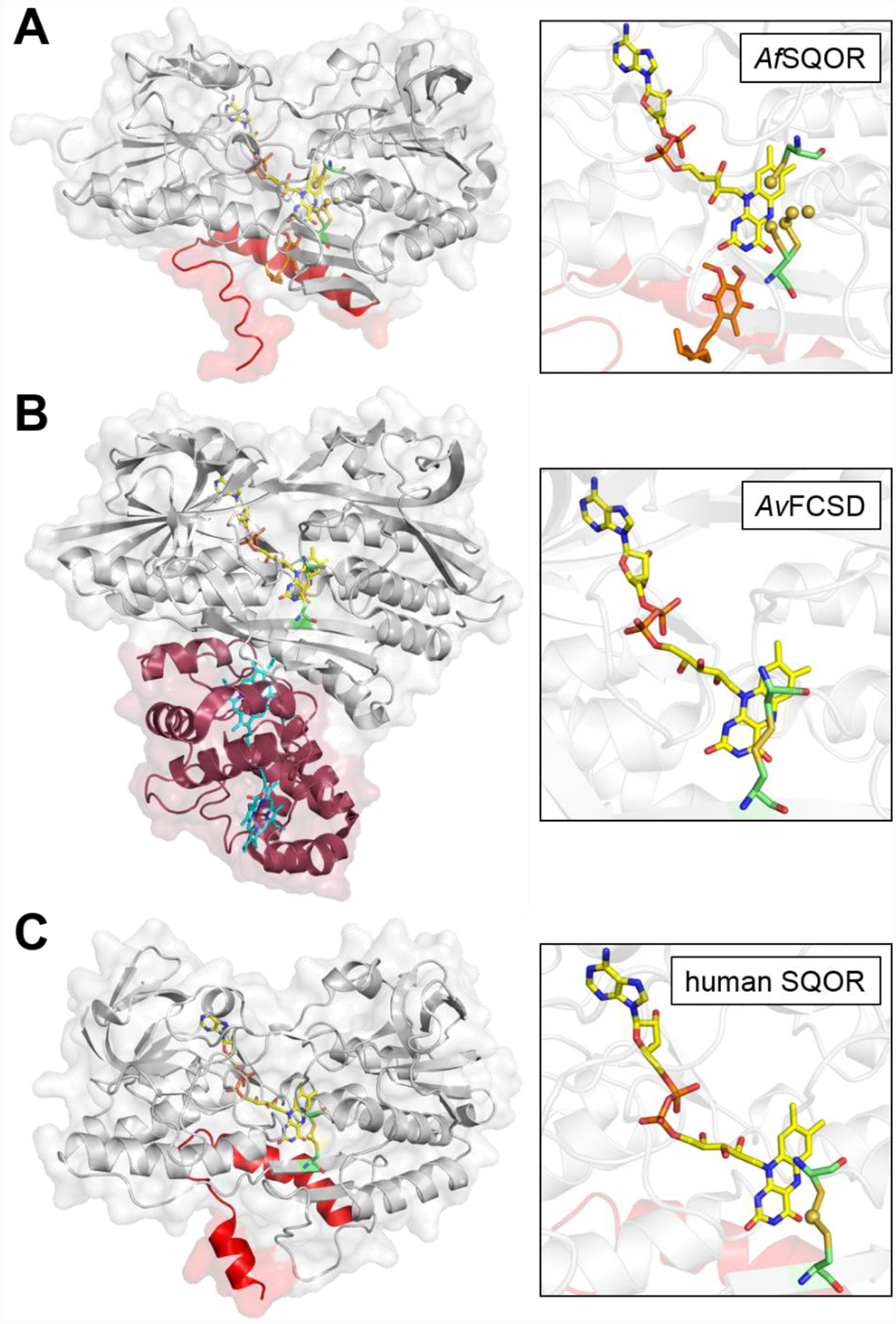
Cartoon and transparent surface overlay representations of A, Acidithiobacillus ferrooxidans SQOR (AfSQOR, PDB ID: 3T31); B, Allochromatium vinosum FCSD (AvFCSD, PDB ID: 1FCD); and C, human SQOR (PDB ID: 6OI5). The amphipathic helices of membrane-anchored AfSQOR and human SQOR are depicted in red, and the diheme cytochrome subunit of cytosolic AvFCSD is depicted in burgundy. Yellow sticks represent the FAD cofactor in each structure. The CoQ analog decylubiquinone in AfSQOR is shown in orange stick display and the dual heme cofactors in the cytochrome subunit of AvFCSD in cyan. An enlarged view of the active sites is shown in each panel on the right, with the redox active cysteines displayed in green sticks. Sulfane sulfur atoms in AfSQOR and bridging the active site cysteines in human SQOR, are shown as yellow spheres.
