Figure 3.
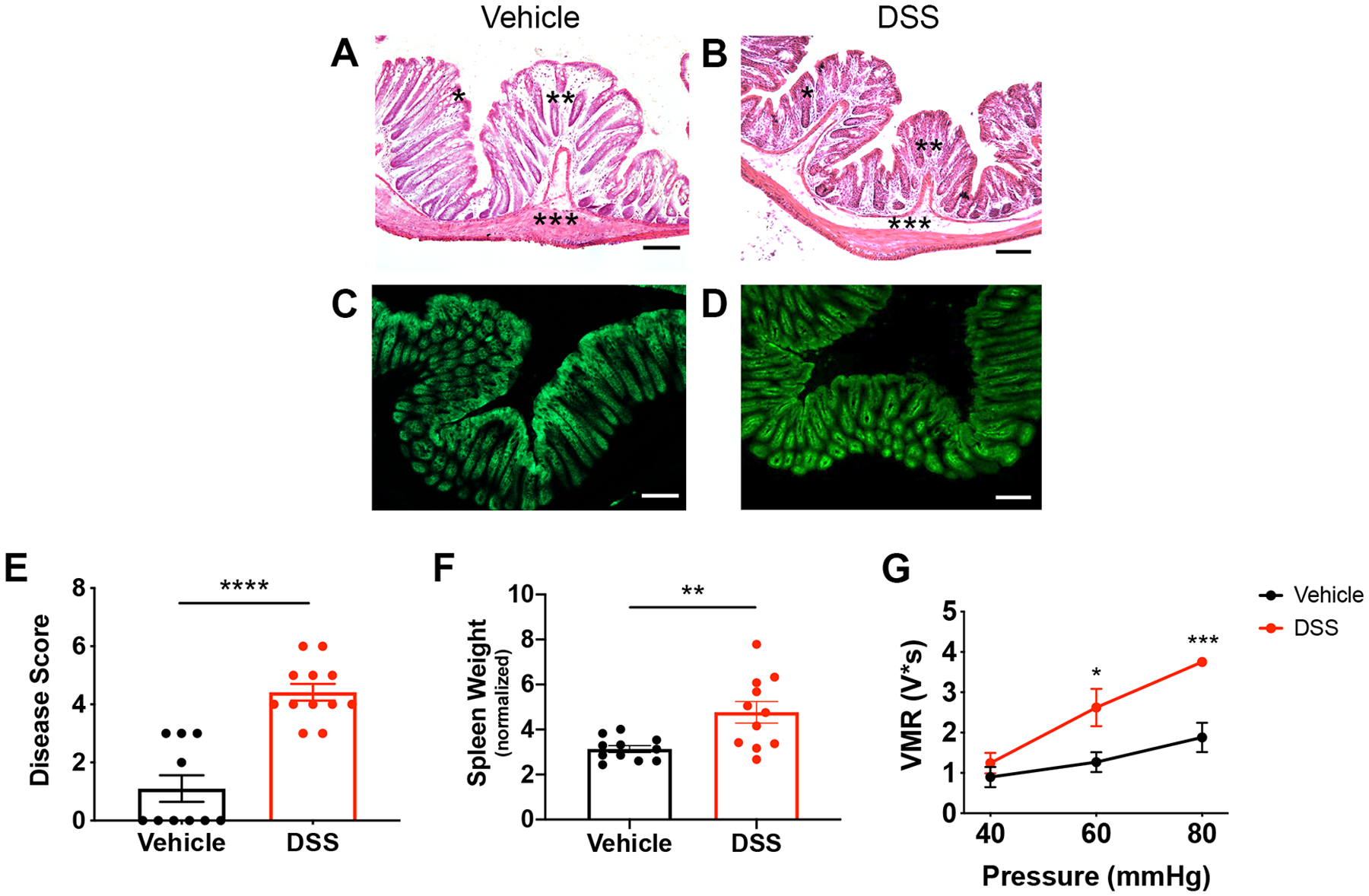
DSS treatment induces colon inflammation and visceral hypersensitivity. Mice were administered 3% DSS for 5 days or vehicle treatment. Histopathological analysis of vehicle-treated (A) vs. DSS-treated (B) colons showed that the DSS protocol did not cause a notable loss in lining epithelial cells. There was a depletion of goblet cells (indicated by *), increase in infiltrating cells (indicated by **), and thickening of the submucosal layer (indicated by ***). Fluorescent images of vehicle-treated (C) and DSS-treated (D) Vil-Arch colons showed comparable EGFP expression, indicating that Arch-EGFP expression is not ablated after DSS treatment. E) DSS-treated mice had a higher inflammation disease score, indicating fewer mucous-secreting goblet cells, more infiltrating cells, and expansion of the submucosa (n = 10 in vehicle-treated group and n = 12 in DSS-treated group; p < 0.0001, unpaired t-test). F) DSS-treated mice also had significantly higher spleen weights indicating inflammation n = 11 in vehicle-treated group and n = 11 in DSS-treated group; p = 0.007, unpaired t-test with Welch’s correction. G) Visceromotor responses (VMRs) to colorectal distension (CRD) were measured in vehicle- and DSS-treated mice. Mice received multiple CRD pressures (40, 60, and 80 mmHg) and showed significantly greater VMRs at 60 (p = 0.01) and 80 mmHg (p < 0.001, two-way ANOVA with Holm-Sidak test; n = 5 mice per group). Scale bars = 100 μ;M.
