Abstract
The risk of left ventricular (LV) and right ventricular (RV) maladaptation after surgery for isolated primary mitral regurgitation (PMR) is poorly defined. We aimed to evaluate LV and RV contractile function using speckle-tracking analysis alongside with quantification of exercise tolerance in patients with PMR after mitral valve surgery. All consecutive patients with symptomatic PMR undergoing mitral valve surgery between July 2015 and May 2017 were prospectively enrolled. Sequential echocardiographic studies along with clinical assessment were performed before and three months after surgery. Mean age in 138 patients was 65.8 ± 12.7 years, 48.2% (66) of whom were female. Mean LV ejection fraction decreased from 57 ± 12% to 50 ± 11% (p < 0.001), LV global longitudinal strain deteriorated from −19.2 ± 4.1% to −15.7 ± 3.8% (p < 0.001), and mechanical strain dispersion increased from 88 ± 12 to 117 ± 115 ms (p = 0.004). There was a reduction in tricuspid annulus plane systolic excursion from 22 ± 5 mm to 18 ± 4 mm (p < 0.001), as well as a slight deterioration of RV free wall mean longitudinal strain from −16.9 ± 5.6% to −15.7 ± 4.1% (p = 0.05). The rate of moderate to severe tricuspid regurgitation significantly decreased (p < 0.005). Regarding exercise tolerance, the New York Heart Association class improved (p < 0.001) and the walking distance increased (p < 0.001). During mid-term follow up after surgery for PMR, a deterioration of LV and RV contractile function measures could be observed. However, the clinical status, LV dimensions, and concomitant tricuspid regurgitation improved which in particular imply more effective RV contractile pattern.
Keywords: Mitral valve surgery, Strain analysis, Myocardial adaptation
Introduction
Primary mitral regurgitation (PMR) due to mitral valve degeneration is the most common etiology in patients undergoing mitral valve surgery [1]. Surgical mitral valve repair or replacement, if repair is unfeasible, is the treatment of choice in case of symptomatic severe PMR [2].
Yet, patients with mitral regurgitation are often referred too late for surgery due to alleged preserved left ventricular (LV) function in echocardiographic controls [3]. Due to the load dependence of standard echocardiographic parameters which are used for the assessment of LV function, LV ejection fraction may substantially overestimate myocardial performance [4, 5].
However, the risk of functional LV maladaptation, the reaction of right ventricular (RV) function, and the resulting clinical implications after mitral valve surgery for isolated mitral regurgitation are poorly defined [6]. On the other side, evaluation of RV function, particularly after cardiac surgery, is challenging due to the complexity of RV geometry, the high RV sensitivity to hemodynamical changes and ventricular interdependence [7].
Speckle-tracking based myocardial deformation analysis has meanwhile become an established method to evaluate myocardial function. Speckle-tracking based assessment of longitudinal strain is independent of the insonation angle, and can be used retrospectively on digitally archived standard grey-scale images [8].
Hence, we aimed to evaluate LV and RV contractile function using longitudinal strain by speckle-tracking analysis together with the clinical status of patients with isolated PMR before and 3 months after mitral valve surgery.
Methods
Assessment of exercise tolerance by the New York Heart Association (NYHA) classification alongside, the 6-min walking test and echocardiographic examinations were prospectively performed before and 3 months after surgery in all consecutive patients with severe PMR who underwent isolated mitral valve surgery between July 2015 and May 2017. The decision for surgical treatment was made after heart team discussion for each case individually. The study was approved by local Ethics Committee of Ruhr University of Bochum and carried out in accordance with the Declaration of Helsinki. All data were included in a database, which is registered at www.clinicaltrials.gov (NCT02296710).
Standard echocardiography
All study participants underwent standard transthoracic echocardiography (EPIQ seven, Philips Electronics, Netherlands). The echo studies were performed by highly qualified medical staff and analysed by the same echocardiographer with long-time experience. The analyses and grading of the mitral regurgitation were performed according to the recommendations of the American and European Societies of Echocardiography [9, 10].
In cases with irregular cardiac rhythm (e.g. atrial fibrillation, frequent atrial or ventricular ectopy) at least five loops were recorded and the average values has been provided.
LV ejection fraction was assessed using the Simpson´s method. LV stroke volume was calculated by subtraction of the LV end-systolic volume from the end-diastolic volume. The Nyquist-limit was placed around 50–60 cm/s in color Doppler settings.
To characterize RV function, tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change (RV-FAC) were measured alongside with RV free wall longitudinal strain analysis.
Strain analyses
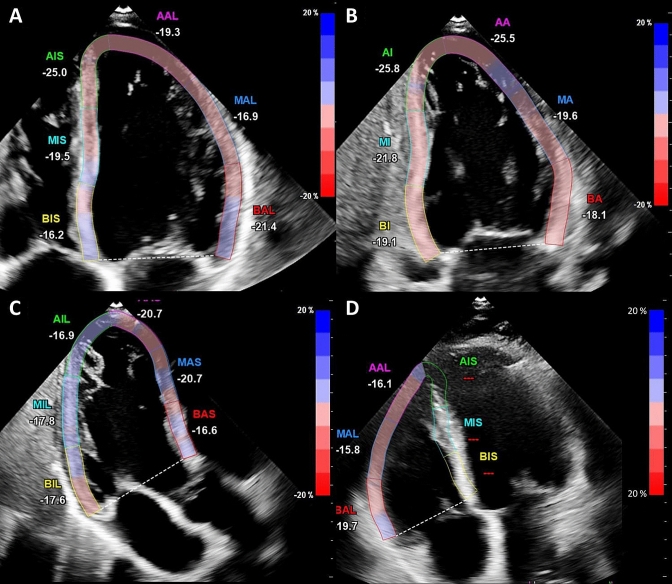
LV global longitudinal strain (GLS) was assessed as previously described using the speckle-tracking algorithm provided within the QLAB system (QLAB Version 10.2) [11]. Through three apical views (four-chamber view, three-chamber view, two-chamber view) the end-diastolic frame was selected and the endocardial contour was tracked manually (Fig. 1a-c).
Fig. 1.
Strain analysis of the left and right ventricle Apical four a two b three chamber view c and a right ventricular focus view d were used for strain analysis. An end-diastolic frame was selected for the left ventricle (with the interventricular septum) and for the right ventricular free wall (without the interventricular septum), and the endocardial contour was manually tracked. The other frames were automatically tracked and corrected, if necessary. After verification the longitudinal strain was automatically calculated on average and regionally
RV free wall longitudinal strain assessment was performed using a RV focused view with optimized RV endocardial borders according to the recommendations of the European and American Societies of Echocardiography (Fig. 1d) [12]. The other frames of the cineloop were tracked automatically and adjusted manually, if needed. Additionally, strain dispersion was documented for each LV segment. Mechanical strain dispersion was calculated as the difference between the highest and the lowest value from time to peak strain assessed through the three apical planes [13].
Statistical analysis
Statistical analysis was performed using the SPSS-Software (Version 21, IBM Corporation, Armonk, NY, USA). Continuous variables are reported as mean ± standard deviation. Categorical variables are presented as frequencies and percentages. Baseline data were validated for normal distribution using the Kolmogorov–Smirnov method. Student’s T-test for unpaired and paired parametric samples or their analogues for nonparametric samples (Mann–Whitney and Wilcoxon signed rank) or the chi-square test were performed for group comparisons. A p-value < 0.05 was considered significant for all comparisons.
Results
A total of 156 consecutive patients with primary mitral regurgitation were admitted and evaluated for mitral valve repair between July 2015 and May 2017. Five of them also required myocardial revascularization and four patients presented with a combined valve disease which had to be addressed. eight patients refused participation in the study and one patient was found to suffer from mitral valve endocarditis. Finally, 138 patients were included in the analyses. The baseline characteristics including parameters for mitral regurgitation severity are shown in Tables 1 and 2. Patients’ mean age was 65.8 ± 12.7 years, and 66 (47.8%) of them were female. Mean EuroScore II was 2.6 ± 2.8%, defining a low to intermediate perioperative risk. Mean LV ejection fraction was 57 ± 12%, and degree of mitral regurgitation was characterized by an effective regurgitant volume of 43 ± 3 mm2, a regurgitant volume of 67 ± 7 ml and a mean biplane vena contracta of 7.3 ± 0.5 mm. Out of the entire group 95 patients (68.9%) underwent mitral valve repair and 43 (31.1%) valve replacement.
Table 1.
Collective-wide baseline characteristics
| Baseline characteristics (n = 138) | |
|---|---|
| Age | 65.8 ± 12.73 (68) |
| Female | 47.8% (66) |
| Coronary artery disease | 10.9% (15) |
| Body mass index [kg/m2] | 26.4 ± 4.3 |
| EuroScore I [%] | 8.6 ± 8.5% |
| EuroScore II [%] | 2.6 ± 2.8% |
| Peripheral artery disease | 4.3% (6) |
| Stroke | 11.6% (16) |
| Diabetes mellitus | 8.7% (12) |
| Renal insufficiency | 10.1% (14) |
| Chronic obstructive pulmonary disease | 7.2% (10) |
| Left bundle branch block | 2.2% (3) |
| History of myocardial infarction | 4.3% (6) |
| History of percutaneous coronary intervention | 5.1% (7) |
| History of cardiac surgery | 11.6% (16) |
| Atrial fibrillation | 34.8% (48) |
| Pacemaker | 3.6% (5) |
Table 2.
Baseline echocardiographic parameters of the whole collective
| Baseline echocardiographic parameters | |
|---|---|
| MV PISA radius adjusted to Nyquist limit 30–40 cm/s [mm] | 10 ± 3 |
| MR vena contracta [mm] | 7.3 ± 0.5 |
| MR effective regurgitant orifice area [mm2] | 43 ± 3 |
| MR regurgitant volume [ml] | 67 ± 7 |
| LA maximal diameter length [mm] | 66 ± 12 |
| LA maximal diameter width [mm] | 55 ± 1 |
| LA volume [ml] | 137 ± 74 |
| LA volume index [ml/m2] | 72 ± 30 |
| LV EF Simpson [%] | 57 ± 12 |
MV mitral valve, MR mitral regurgitation, LA left atrium, LV left ventricle, EF ejection fraction, PISA proximal isovelocity surface area
Details of echocardiographic parameters before and after surgery are presented in Table 3 (left ventricle) and Table 4 (right ventricle). Three months after surgery, 121 patients (87.7%) had no residual MR and in 17 patients (12.3%) only trivial MR was detectable.
Table 3.
Morphological and functional changes of the left ventricle
| Echocardiographic parameters | Before surgery | 3 months after surgery | p-value |
|---|---|---|---|
| LA maximal diameter length [mm] | 66 ± 11 | 55 ± 10 | < 0.001 |
| LA maximal diameter width [mm] | 55 ± 9 | 50 ± 8 | < 0.001 |
| LA volume [ml] | 137 ± 74 | 97 ± 51 | < 0.001 |
| LA volume index [ml/m2] | 72 ± 30 | 47 ± 25 | < 0.001 |
| LVEDD [mm] | 58 ± 7 | 55 ± 8 | < 0.001 |
| LVESD [mm] | 4.0 ± 7 | 4 ± 9 | 0.5 |
| Septum thickness [mm] | 9 ± 2 | 8 ± 4 | 0.4 |
| Post wall thickness [mm] | 9 ± 2 | 9 ± 1 | 0.6 |
| FS [%] | 31 ± 9 | 28 ± 11 | 0.003 |
| LV end-diastolic volume [ml] | 157 ± 57 | 138 ± 51 | < 0.001 |
| LV end-systolic volume [ml] | 67 ± 31 | 70 ± 35 | 0.2 |
| LV EF Simpson [%] | 57 ± 12 | 50 ± 11 | < 0.001 |
| Stroke volume [ml] | 93 ± 39 | 68 ± 25 | < 0.001 |
| GLS [%] | −19.2 ± 4.1 | −15.7 ± 3.8 | < 0.001 |
| Mechanical strain dispersion [msec] | 88 ± 12 | 117 ± 115 | 0.004 |
LA left atrial, LV left ventricular, LVEDD left ventricular end-diastolic diameter, LVESD left ventricular end-systolic diameter, FS fractional shortening, GLS global longitudinal strain, EF ejection fraction
Table 4.
Morphologic and functional changes of the right ventricle
| Echocardiographic parameters | Before surgery | 3 months after surgery | p-value |
|---|---|---|---|
| RA maximal diameter length [mm] | 57 ± 11 | 54 ± 10 | 0.005 |
| RA maximal diameter width [mm] | 45 ± 10 | 44 ± 8 | 0.9 |
| RA volume [ml] | 78 ± 54 | 74 ± 45 | 0.8 |
| RV maximal diameter [mm] | 43 ± 9 | 43 ± 8 | 0.9 |
| TAPSE [mm] | 22 ± 5 | 18 ± 4 | < 0.001 |
| RV end-diastolic area [mm2] | 19 ± 6 | 19 ± 7 | 0.4 |
| RV end-systolic area [mm2] | 11 ± 5 | 11 ± 4 | 0.2 |
| FAC [%] | 42 ± 12 | 42 ± 11 | 0.7 |
| RV basal segment strain [%] | −15.6 ± 5.5 | −14.9 ± 4.5 | 0.3 |
| RV middle segment strain [%] | −18.3 ± 7.0 | −16.2 ± 4.7 | 0.004 |
| RV apical segment strain [%] | −17.1 ± 6.8 | −15.9 ± 5.0 | 0.4 |
| RV mean strain [%] | −16.9 ± 5.6 | −15.7 ± 4.1 | 0.05 |
| Tricuspid regurgitation degree [I-III] |
33.6% 0 46.0% I 15.1% II 5.3% III |
44.3% 0 50.4% I 4.4% II 0.9% III |
< 0.001 |
| TV PISA radius adjusted to Nyquist limit 30–40 cm/s [mm] | 3.6 ± 5.0 | 2.8 ± 4.0 | 0.04 |
| TR vena contracta [mm] | 1.9 ± 0.3 | 1.1 ± 0.3 | 0.03 |
FAC fractional area change, RA right atrial, RV right ventricular, TAPSE tricuspid annular plane systolic excursion, TR tricuspid regurgitation, TV tricuspid valve, PISA proximal isovelocity surface area
LV end-diastolic volume markedly decreased from 157 ± 57 ml to 138 ± 51 ml (p < 0.001) following valve surgery, while the other morphological parameters such as end-systolic diameter, septal thickness and posterior wall thickness did not change.
Compared to baseline examinations, LV ejection fraction decreased from 57 ± 12% to 50 ± 11% (p < 0.001) while LV GLS deteriorated from −19.2 ± 4.1% to −15.7 ± 3.8% (p < 0.001). Additionally, strain dispersion increased from 88 ± 12 ms to 117 ± 115 ms (p = 0.004).
With respect to RV function, tricuspid annular plane systolic excursion (TAPSE) was reduced from 22 ± 5 mm to 18 ± 4 mm (p < 0.001). RV free wall mean strain also showed a slight although borderline significant deterioration (from −16.9 ± 5.6% to −15.7 ± 4.1%; p = 0.05). However, tricuspid regurgitation improved after mitral valve surgery (Fig. 2b). While 20.4% of the patients had a moderate to severe TR before mitral valve surgery, this figure decreased to 5.3% 3 months after surgery with consecutively decreasing RA diameter (Table 4).
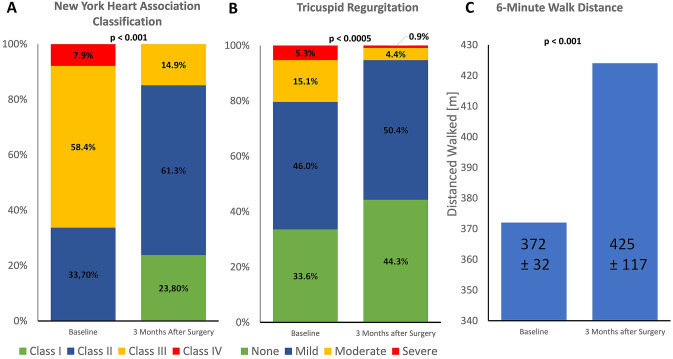
Fig. 2.
Clinical Improvement and Reduction of Tricuspid Regurgitation after Mitral Valve Surgery Patients with symptomatic primary mitral regurgitation presented a noticeably clinical improvement accompanying the reduction of tricuspid regurgitation as a sign for economized right ventricular function. The New York Heart Association class improved significantly. At baseline 66.3% were in NYHA class III or IV. 3 months after surgery 85.2% were in NYHA class I or II (p < 0.001) a The rate of moderate to severe tricuspid regurgitation decreases from 20.4% to 5.3% b The walking distance in the 6-min walking test increased from 372 ± 32 m to 425 ± 117 m (p < 0.001) (c)
Regarding exercise tolerance, NYHA classification (at baseline 66.3% were in NYHA class III or IV, 3 months after surgery 85.2% were in NYHA class I or II; p < 0.001) and walking distance in the 6-min walking test (372 ± 32 m to 425 ± 117 m; p < 0.001) improved significantly (Fig. 2a, c).
Discussion
Due to the poorly defined risk for ventricular dysfunction after mitral surgery and its clinical impact, we evaluated the adaptation of the left and right ventricle after surgical mitral valve treatment in patients with severe mitral regurgitation and the clinical status before and 3 months after surgery.
Left ventricular dysfunction after mitral valve surgery
Mean LV GLS in our patients was −19.2% at baseline and showed a deterioration after mitral valve surgery as an indicator for LV dysfunction. This is in accordance with the retrospective observation of Witkowski et al. who described a GLS worse than −19.9% as an independent predictor for LV dysfunction in severe primary mitral regurgitation [14].
Hiemsatra et al. described LV GLS as independently associated with all-cause mortality and cardiovascular events in a cohort of 593 patient who underwent mitral valve surgery with a median follow-up of 6.4 years, (Hazard ratio 1.13; 95% confidence interval: 1.06 to 1.21 p < 0.001). In this study, LV-EF and LV GLS showed a similar deterioration of the contractile function (3). In a retrospectively analysed cohort of 506 patients with a wide range of cardiac comorbidities and a median follow-up of 3.5 years, Kim et al. postulated GLS to better predict cardiac events and all-cause mortality than standard echocardiographic parameters (Multivariate Cox models HR 1.229 95% CI: 1.135 to 1.331; p < 0.001). The authors concluded this measure to be helpful to estimate the optimal timing for mitral valve surgery [15]. Interestingly, mechanical strain dispersion also increases after mitral valve surgery (Table 4). Prolonged mechanical strain dispersion is a sign for heterogeneity of systolic myocardial contraction due to the development of fibrosis formation and is associated with cardiac arrhythmias [16]. Therefore, strain dispersion could provide important information about cardiac remodeling during patient evaluation for mitral valve surgery [17].
However, despite functional impairment of the left ventricle, the patients showed pronounced clinical improvement in NYHA class and 6-min walking distance (Fig. 2a, c). Moreover, LA and LV diameter and volumes decreased after mitral valve surgery demonstrating a relevant reverse remodelling. By eliminating the regurgitation fraction of overall stroke volume, LV enlargement receded allowing for normal stress shortening [18, 19]. However, since stroke volume and ejection fraction are required for antegrade flow only, myocardial performance is optimized and economized [20] whereas, according to our results, at least a temporary postoperative medical therapy to support myocardial unloading and reverse remodelling is suggested.
Right ventricular dysfunction after mitral valve surgery
Mitral regurgitation leads to a volume overload of the LA [18]. The LA is initially able to keep the pressure stable through its enlargement, but over time the pressure in the pulmonary venous system increases which eventually leads to an increased pulmonary artery pressure [7].
In the absence of volume overload after surgery, the pressure in the pulmonary vascular bed and consecutively in the right ventricle decreases. Right ventricular dimensions and functional tricuspid regurgitation are consecutively reduced [7]. However, as on the left side, some measures of RV function decreased. While FAC did not change, RV free wall strain and TAPSE were reduced. This deterioration is probably explained by geometric changes of the RV due to pericardial incision and the loss of pericardial support [21]. Depending on the pericardial incision and the surgical access path, parameters for the longitudinal RV function can show a decrease, despite overall normal global right ventricular function [21].
Another aspect is the reduced mobility of the septum due to the increased LV impairment. In addition, the incompletely understood cardioplegia effect may have played a role [3, 22–24]. The septal wall is involved in the mechanism of “squeezing out” the right ventricle. Together with the apex, the septal wall serves as an abutment to counteract the tension of the bellow-type right ventricle, and thus transports the blood towards the pulmonary arteries. About 24% of the RV function is taken over by the septal wall [7].
Our mid-term follow-up data on exercise tolerance demonstrate a clear clinical improvement, which implies an economization and higher effectiveness of RV myocardial performance. Accordingly, tricuspid regurgitation also improved after surgery probably because of improved hemodynamic and lack of volume overload which is also a sign of recovered clinical status [25, 26].
Limitations
The study is descriptive and not designed to explain the phenomena it observes and can therefore only generate hypotheses. In addition, further studies should investigate whether and to what extent the deteriorated function parameters persist during longer-term follow-up and whether this has a long-term impact on survival.
Conclusion
During mid-term follow up after surgery for PMR, a deterioration of LV and RV contractile function measures could be observed. However, the clinical status, LV dimensions, and concomitant tricuspid regurgitation improved significantly which in particular imply more effective RV contractile pattern.
Author contributions
All authors have made substantial contributions to the manuscript, are responsible for the contents, and have read and approved the manuscript for submission to the International Journal of Cardiovascular Imaging.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by the Medical Faculty, Ruhr-Universität Bochum, Germany (FoRUM programme F811-14).
Data Availability
All presented data are available and will be issued if necessary.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–30.e3. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Hiemstra YL, Tomsic A, van Wijngaarden SE, Palmen M, Klautz RJM, Bax JJ, et al. Prognostic value of global longitudinal strain and etiology after surgery for primary mitral regurgitation. JACC Cardiovasc Imag. 2020;13:577–585. doi: 10.1016/j.jcmg.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Lee R, Haluska B, Leung DY, Case C, Mundy J, Marwick TH. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart. 2005;91:1407–1412. doi: 10.1136/hrt.2004.047613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starling MR, Kirsh MM, Montgomery DG, Gross MD. Impaired left ventricular contractile function in patients with long-term mitral regurgitation and normal ejection fraction. J Am Coll Cardiol. 1993;22:239–250. doi: 10.1016/0735-1097(93)90840-W. [DOI] [PubMed] [Google Scholar]
- 6.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Del Rio JM, Grecu L, Nicoara A. Right ventricular function in left heart disease. Semin Cardiothorac Vasc Anesth. 2019;23:88–107. doi: 10.1177/1089253218799345. [DOI] [PubMed] [Google Scholar]
- 8.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 9.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imag. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 10.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 11.Dimitriadis Z, Scholtz S, Ensminger S, Wiemer M, Fischbach T, Scholtz W, et al. Left ventricular adaptation after TAVI evaluated by conventional and speckle-tracking echocardiography. Int J Cardiol. 2017;228:633–637. doi: 10.1016/j.ijcard.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imag. 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 13.Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imag. 2013;6:841–850. doi: 10.1016/j.jcmg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imag. 2013;14:69–76. doi: 10.1093/ehjci/jes155. [DOI] [PubMed] [Google Scholar]
- 15.Kim HM, Cho GY, Hwang IC, Choi HM, Park JB, Yoon YE, et al. Myocardial strain in prediction of outcomes after surgery for severe mitral regurgitation. JACC Cardiovasc Imag. 2018;11:1235–1244. doi: 10.1016/j.jcmg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imag. 2010;3:247–256. doi: 10.1016/j.jcmg.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E, et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imag. 2016;17:613–621. doi: 10.1093/ehjci/jew005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candan O, Hatipoglu Akpinar S, Dogan C, Demirkiran A, Dindar B, Bayram Z, et al. Twist deformation for predicting postoperative left ventricular function in patients with mitral regurgitation: a speckle tracking echocardiography study. Echocardiography. 2017;34:422–428. doi: 10.1111/echo.13462. [DOI] [PubMed] [Google Scholar]
- 19.Gaasch WH, Shah SP, Labib SB, Meyer TE. Impedance to retrograde and forward flow in chronic mitral regurgitation and the physiology of a double outlet ventricle. Heart. 2017;103:581–585. doi: 10.1136/heartjnl-2016-309747. [DOI] [PubMed] [Google Scholar]
- 20.Imasaka K, Tomita Y, Tanoue Y, Tominaga R, Tayama E, Onitsuka H, et al. Early mitral valve surgery for chronic severe mitral regurgitation optimizes left ventricular performance and left ventricular mass regression. J Thorac Cardiovasc Surg. 2013;146:61–66. doi: 10.1016/j.jtcvs.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Unsworth B, Casula RP, Kyriacou AA, Yadav H, Chukwuemeka A, Cherian A, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2010;159:314–322. doi: 10.1016/j.ahj.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 23.Zanobini M, Saccocci M, Tamborini G, Veglia F, Di Minno A, Poggio P, et al. Postoperative echocardiographic reduction of right ventricular function: is pericardial opening modality the main culprit? BioMed Res Int. 2017;2017:4808757. doi: 10.1155/2017/4808757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roshanali F, Yousefnia MA, Mandegar MH, Rayatzadeh H, Alinejad S. Decreased right ventricular function after coronary artery bypass grafting. Tex Heart Inst J. 2008;35:250–255. [PMC free article] [PubMed] [Google Scholar]
- 25.Katsi V, Raftopoulos L, Aggeli C, Vlasseros I, Felekos I, Tousoulis D, et al. Tricuspid regurgitation after successful mitral valve surgery. Interactive CardioVasc Thorac Surg. 2012;15:102–108. doi: 10.1093/icvts/ivs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamborini G, Fusini L, Muratori M, Gripari P, Ghulam Ali S, Fiorentini C, et al. Right heart chamber geometry and tricuspid annulus morphology in patients undergoing mitral valve repair with and without tricuspid valve annuloplasty. Int J Cardiovasc Imag. 2016;32:885–894. doi: 10.1007/s10554-016-0846-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All presented data are available and will be issued if necessary.