Highlights
-
•
We applied lesion network mapping analysis to three autoscopic phenomena.
-
•
Connectivity with temporo-parietal junction was a common core alteration.
-
•
Additional patterns of functional connectivity defined each form of autoscopic phenomena.
-
•
We link autoscopic phenomena to different sensorimotor and self-related networks.
Keywords: Lesion network mapping, Multisensory processing, Bodily self-consciousness, Temporo-parietal junction
Abstract
Objective
Autoscopic phenomena (AP) are illusory own body reduplications characterized by the visual perception of a second own body in extrapersonal space, and include three main forms: autoscopic hallucination (AH), heautoscopy (HAS) and out-of-body-experience (OBE). Past research showed that lesions were heterogeneously distributed and affected many different brain regions within and across patients, while small case series suggested that AP lesions converge in temporo-parietal and parieto-occipital cortex. As only few studies investigated each form of AP separately, it remains unknown whether the three AP are characterized by common and distinct brain mechanisms.
Methods
Here, we applied lesion network analysis in 26 neurological AP patients and determined their common and distinct functional connectivity patterns.
Results
We report that all localize to a single common brain network at the bilateral temporo-parietal junction, further associated with specific patterns of functional connectivity, defining each type of AP. OBE resulted from a brain network connected to bilateral angular gyrus, right precuneus, and right inferior frontal gyrus, differing from AH with a brain network connected to bilateral precuneus, inferior temporal gyrus, and cerebellum. HAS resulted from a brain network connected to left inferior frontal gyrus, left insula and left parahippocampus.
Conclusion
The present data identify the temporo-parietal junction as the common core region for AP and show that each form of AP recruits additional specific networks, associated with different sensorimotor and self-related sub-networks.
1. Introduction
Autoscopic phenomena (AP) are illusory multisensory own body reduplications that occur in various neuropsychiatric conditions and the healthy population (Brugger et al., 1999, Devinsky et al., 1989, Hécaen and Ajuriaguerra, 1952). AP are characterized by the illusory visual perception of one’s own body in extrapersonal space, are classified among disorders of the body schema (Devinsky et al., 1989, Brugger and Landis, 1997) and have recently been the target of neuroscientific investigations due to their relevance for self and self-consciousness and related multisensory processing (Blanke, 2012).
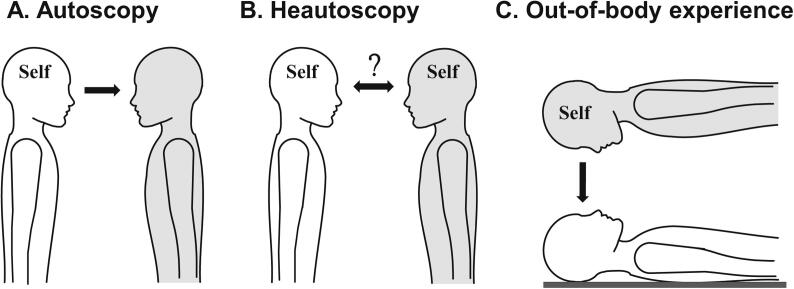
AP include three main forms: autoscopic hallucination (AH), heautoscopy (HAS), and out-of-body-experience (OBE) (Devinsky et al., 1989, Brugger and Landis, 1997, Brugger, 2002, Blanke et al., 2004). AH (Fig. 1A) have been associated with damage or interference to occipital, occipito-temporal, or occipito-parietal cortex (Zamboni et al., 2005, Bolognini et al., 2011, Blanke and Castillo, 2007, Maillard et al., 2004) and lesion overlap analysis highlighted damage in right occipito-parietal cortex, including the superior occipital gyrus and the cuneus (Heydrich and Blanke, 2013). Single case reports and a meta-analysis regrouping several neurological patients with OBE (Fig. 1C) suggested a right hemispheric dominance and involvement of the parietal and temporal cortex (Blanke et al., 2004, Maillard et al., 2004, Brandt et al., 2005, Blanke and Mohr, 2005), whereas lesion overlap analysis in OBE patients converged on damage to the right angular gyrus and the posterior superior temporal gyrus (pSTG) (Ionta et al., 2011). HAS (Fig. 1B) was less investigated compared to OBE and AH, and, although case reports linked HAS to damage in temporal and parietal cortex (Blanke and Mohr, 2005, Brugger et al., 1994, Brugger et al., 2006, Anzellotti et al., 2011, Tadokoro et al., 2006, Arias et al., 2007), lesion overlap analysis in HAS patients suggested a different brain region and converged on the left insula (Heydrich and Blanke, 2013).
Fig. 1.
Autoscopic phenomena (AP). Phenomenology of the different AP: autoscopic hallucination (A), heautoscopy (B) and out-of-body experience (C). A. During AH, patients report seeing an autoscopic body in extrapersonal space from their habitual body-centered self-location and perspective (indicated by Self). B. During HAS, patients report alternating self-location and first-person perspective between the physical body and the autoscopic body. C. In OBE, patients report disembodiment (i.e. the experience of being located outside the physical body) and to be located with an elevated position and perspective. Self in each depiction represents experienced self-location and the arrow represents the experienced direction of the first-person perspective, for each AP separately (for more detail see main text). The grey body represents the location of the illusory autoscopic body.
Despite these lesion overlaps that differed for each AP, most of the studies mentioned above found that brain damage was heterogeneously distributed and affected many different brain regions within and across patients, suggesting that AP, despite being associated with damage to one or several posterior brain regions, may emerge from dysfunctional brain networks rather than only damage to a single or limited number of brain regions. Moreover, the number of tested patients remained low and AP occur in many different patient populations (Devinsky et al., 1989, Blanke et al., 2004, Ionta et al., 2011), some of which are not associated with focal brain damage, several cases have been linked to epilepsy, compatible with propagation of ictal activity within more extended networks. Based on these findings we hypothesized that AP may be associated with alterations in functional brain connectivity as measured by fMRI (Brugger et al., 1999, Hécaen and Ajuriaguerra, 1952). Lesion network analysis may uncover brain networks associated with specific neurological symptoms (Boes et al., 2015) and allows to determine whether heterogeneously distributed lesions causing the same symptom are part of the same network. Lesion network analysis has been successfully applied to complex symptoms such as delusional misidentification syndromes (Darby et al., 2017).
Here, we applied lesion network analysis to 26 neurological AP patients and sought to investigate the networks of each AP, determining their common and distinct networks. We hypothesized (1) that AH, OBE and HAS would share parts of their networks because they all share an altered self-representation characterized by an illusory reduplication of their body (Brugger and Landis, 1997, Blanke et al., 2004) and (2) that each form of AP would recruit additional specific networks, based on previous work associating each form with different sensory, motor, and cognitive mechanisms (Blanke et al., 2004, Maillard et al., 2004, Heydrich and Blanke, 2013).
2. Methods
2.1. Patients
The lesion masks of patients experiencing the different AP were taken from previously published studies (Heydrich and Blanke, 2013, Ionta et al., 2011) to which we added one unpublished case with HAS (Supplementary Materials). This lead to a total of 26 patients: seven with AH cases, ten with HAS (Heydrich and Blanke, 2013); nine with OBE (Ionta et al., 2011) (Supplementary Table S1).
During AH, subjects report seeing a second own (autoscopic) body in extrapersonal space, which most often consists of the upper body parts (face and/or torso) that is seen in front-view, as if they were looking in a mirror. The subject’s center of awareness remains within the physical body and they see the world and the autoscopic body from their habitual body-centered visuo-spatial viewpoint (Fig. 1A). Patients do not report feelings of disembodiment (i.e. the conscious experience of being located outside one’s body) that is typical for OBE.
Subjects experiencing OBE also report seeing a second own body in extrapersonal space, but from an elevated visuo-spatial perspective, which is characteristically associated with the conscious experience of disembodiment. The center of awareness during OBE is located at the elevated visuo-spatial perspective and patients may also report vestibular sensations of elevation, floating or flying (Fig. 1C) (Devinsky et al., 1989, Brugger, 2002, Blanke et al., 2004).
HAS is defined as an intermediate AP, between AH and OBE, with elements of disembodiment and perspective changes while subjects also report seeing a second own body in extrapersonal space. Compared to AH, a complete (not only upper) autoscopic body is seen, often in various side- and back-views. Moreover, subjects have difficulties to determine their center of awareness and the origin of the visuo-spatial perspective, which may be experienced at their physical body, at the location of the hallucinated body, or at alternating locations (Fig. 1B). Thus, HAS is often associated with the feeling of bi-location and strong self-identification and close emotional affinity with the autoscopic body (Devinsky et al., 1989, Brugger, 2002, Blanke and Mohr, 2005), which may even persist if the autoscopic body only partly reflects the patient’s visual bodily appearance.
Ethical approval was obtained from the Ethical committee at the University Hospital of Geneva and most of the patients have been reported previously (Heydrich and Blanke, 2013, Ionta et al., 2011) (for details see Supplementary Table S1). One unpublished case from the Clinique Romande de Réadaptation (Sion, Switzerland) was included (Supplementary Materials).
2.2. Lesion network mapping
For each patient we identified the lesion-derived network from each seed region of interest (ROI) following the lesion network mapping approach as described previously (Boes et al., 2015, Darby et al., 2017). The method consists in three main steps: (1) mapping each lesion into standard MNI space, (2) computing its functional connectivity at rest in a normative resting state database of healthy subjects, (3) and overlapping each of the binarized lesion-derived network together.
2.3. MRI acquisition
For this we used the resting state and T1-weighted structural data from 151 healthy participants obtained from the publicly available Enhanced Nathan Kline Institute Rockland Sample (Nooner et al., 2012). All participants were right handed and aged between 19 and 40 years (25.8 ± 5.5 years, 83 females). Scans were acquired with a 3 T Siemens Magneton TrioTim syngo. For the resting sate data, a multiband EPI sequence was used (multiband factor = 4, 64 continuous slices, TR = 1.4 s, TE = 30 ms, flip angle = 65°, slice thickness = 2 mm) and 404 scans were collected. For each participant, an anatomical image was recorded using a T1–weighted MPRAGE sequence (TR = 1.9 s, TE = 2.52 ms, Inversion time = 900 ms, flip angle = 9°, 1 mm isotropic voxels, 176 slices per slab and FOV = 250 mm).
2.4. Image pre-processing
The pre-processing steps were performed using Matlab (R2018b, MathWorks) with Statistical Parametric Mapping (SPM12, Wellcome Trust Centre for Neuroimaging, London). The first four functional scans were discarded from the analysis to allow for magnetic saturation effects therefore the analysis was performed on the 400 scans remaining. The standard pre-processing pipeline was applied and included slice-timing correction, spatial realignment and co-registration of the anatomical images to the mean functional image. The functional and anatomical scans were then normalized to the Montreal Neurological Institute space (MNI space). Finally, the functional scans were spatially smoothed with a 5 mm full-width at half-maximum isotropic Gaussian kernel. The anatomical T1-weighted image was segmented into grey and white matter and cerebrospinal fluid (CSF). The data were filtered with a bandwidth of 0.008 Hz to 0.09 Hz. The six motion parameters and their first-degree derivative were included as nuisance regressors in addition to the bold activity in the white matter and the cerebro-spinal fluid. Subjects with excessive motion were excluded from the analysis, this comprised 25 subjects which had more than 15% of scans affected by movement as calculated by the framewise displacement (Power et al., 2012). In total, 126 subjects were included for the analysis.
2.5. Resting state analysis
The resting state data was analyzed using the CONN-fMRI Functional Connectivity toolbox (v.18.a, http://www.nitrc.org/projects/conn Whitfield-Gabrieli and Nieto-Castanon, 2012). The lesion masks were used as seed ROIs and their mean time course was extracted and correlated to all other brain voxels, limiting our analysis to voxels within the grey matter. Finally, the brain network derived from each seed lesion was threshold at T > 4.25 with p < 0.00005 FWE peak-level whole brain corrected similar to previous literature (Boes et al., 2015, Darby et al., 2017). The lesion network maps were then binarized and overlapped together to determine the regions of shared positive and negative correlations.
Four different lesion network mapping analyses were performed. First, we applied lesion network mapping analysis for each AP separately to identify the brain networks associated with AH (N = 7), HAS (N = 10) and OBE (N = 9); networks were thresholded at 100% of the cases to be as restrictive as possible given the limited number of patients per AP). After these three lesion network mapping analyses for each AP, we next, we applied lesion network mapping analysis to the entire group of patients with AP (N = 26). This was done in order to determine all regions functionally connected to the lesion locations of neurological patients with any type of AP. Here, we applied a more liberal threshold of 90% due to the higher number of patients for the analysis. For all analyses, only clusters larger than 10 voxels were considered. The anatomical regions were labeled according to the AAL atlas (Tzourio-Mazoyer et al., 2002) implemented in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron) and the Anatomy toolbox (Eickhoff et al., 2005).
2.6. Specificity
In a further analysis step, we assessed whether the regions connected for AH, HAS and OBE were specific to each AP. To this aim, we compared the lesion network maps of each AP against all others using a Liebermeister statistical test, using voxel-based lesion symptom mapping (VLSM) (Rorden et al., 2007). This method enabled us to identify those voxels, in which the connectivity was significantly altered in a particular symptom compared to the voxels altered by another symptom. The analysis was performed on the binary maps (separately for positive and negative maps). We corrected for multiple comparisons using FWE correction (p < 0.05 and 1000 permutations). For this analysis, we only considered voxels within 100% of the lesion network map in order to determine only the brain regions specific for each AP.
2.7. Data availability
All data are available from the corresponding authors upon request.
3. Results
3.1. Autoscopic hallucinations (AH)
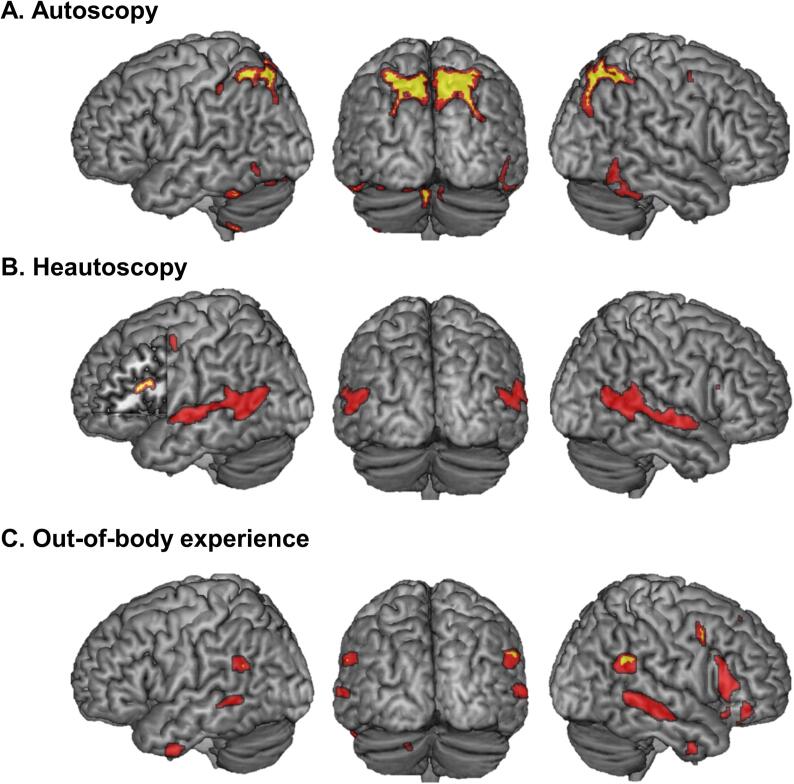
Lesions causing AH were mainly positively connected to a large cluster in bilateral precuneus and adjacent regions in superior occipital cortex and superior parietal cortex, as well as bilateral occipito-temporal cortex (posterior inferior temporal cortex (pITG)) and bilateral cerebellum (Fig. 2A). Several much smaller clusters also showed negative functional connectivity with lesions causing AH (Supplementary Table S2).
Fig. 2.
Lesion derived network for each AP. Brain networks connected to lesions causing autoscopic hallucinations (AH) (A), heautoscopy (HAS) (B) and out-of-body experiences (OBE) (C). Brain networks for each AP are depicted in red. The yellow regions indicate those brain regions that are specific for each AP as compared to the other two AP (Liebermeister test; see methods and results for further detail). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When comparing the AH network maps with the networks maps of the two other AP, we found that bilateral precuneus, cerebellum and pITG were specifically connected to the lesions causing AH, but not in any of the other two forms of AP (Supplementary Table S2).
3.2. Heautoscopy (HAS)
Lesions causing HAS were positively connected to large bilateral clusters in middle and superior temporal gyrus (MTG/STG), parahippocampal gyrus (PHC), inferior temporal gyrus (Fig. 2B). Smaller clusters were found in left inferior frontal gyrus (IFG), left precentral gyrus, and left thalamus (Fig. 2B). The left caudate nucleus was found negatively connected to lesions causing HAS.
When comparing the HAS network maps with the network maps of the other AP, we found that only left hemispheric clusters in the left IFG, left insula and the left parahippocampal gyrus/hippocampus were specific to HAS compared to the other two forms of AP (Supplementary Table S2).
3.3. Out-of-body experiences (OBE)
Lesions causing OBE were all positively connected to bilateral supramarginal gyrus (SMG)/angular gyrus, bilateral posterior MTG and inferior temporal gyrus and to the right precuneus, right IFG, right superior frontal gyrus, right precentral gyrus, and left cerebellum (Fig. 2C). The right caudate nucleus was found negatively connected to lesions causing OBE.
When comparing the OBE network maps with the network maps of the other AP, we found the bilateral angular gyrus, the right IFG, the right precuneus and the left cerebellum to be specific to OBE compared to the other two forms of AP (Supplementary Table S2).
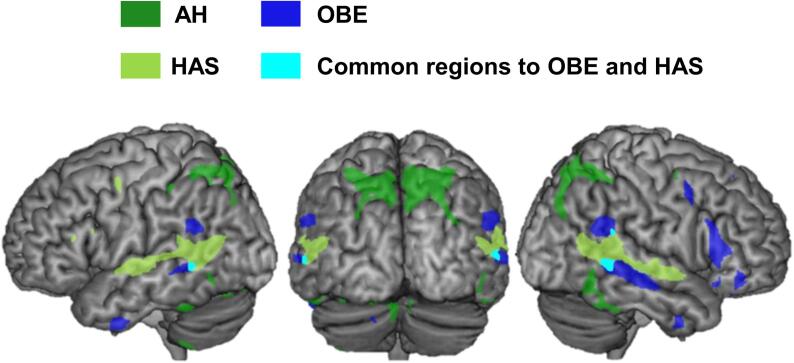
When spatially overlapping the lesion derived network maps of each of the three AP, we observed visually that HAS and OBE had relatively similar brain network connectivity with common bilateral activations of the TPJ, while AH also elicited a bilateral parietal-occipital network (Fig. 3). There was limited overlap across all three AP using this approach. In order to better quantify the common brain regions connected to all AP, we next applied the lesion network mapping analysis to the entire group of patients with AP (N = 26) (independently of each individual form of AP).
Fig. 3.
Overlap of all three AP networks. The brain networks of each AP were overlap and displayed onto a common template brain to reveal common and distinct network components. AH is shown in green, OBE in blue and HAS in light green. Shared brain networks were observed between HAS and OBE (overlap indicated in light blue) in the temporo-parietal junction. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Common lesion network
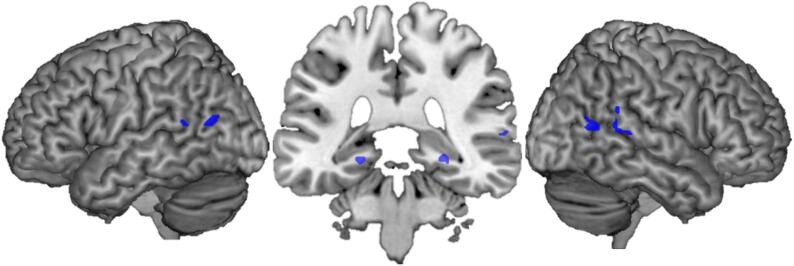
To search for brain regions that were commonly involved in all AP, we considered all AP cases together and applied lesion network mapping analysis to all 26 AP patients. This analysis revealed that lesions causing AP were positively connected to the bilateral TPJ, involving the bilateral posterior MTG, the right posterior STG as well as smaller clusters in the bilateral hippocampus/parahippocampus (Fig. 4) (Supplementary Table S3).
Fig. 4.
Common AP brain regions. 90% of the lesions causing AP were connected to a brain network including bilateral temporo-parietal junction (in bilateral pMTG and right STG) as well as in smaller bilateral clusters in the hippocampus/parahippocampus.
4. Discussion
We investigated the brain networks underlying AP and defined their common and distinct patterns of network connectivity: we found that all AP share common network connections with bilateral TPJ and also provide evidence for specific network connections for each of the three AP. Below we discuss the specific and common AP networks and the relevance of these data for clinical neurology and for neuroscience research on self-consciousness.
4.1. Autoscopic hallucinations (AH)
The present data show that AH-lesions are connected to specific regions consisting of bilateral precuneus, pITG and cerebellum, providing new evidence that AH result from disrupted functional connectivity with a larger cortical and subcortical network, involving self-related and visual brain mechanisms. Concerning visual mechanisms, AH is a complex structured visual hallucination of the patient’s face or upper body and often associated with more elementary visual hallucinations (Blanke and Mohr, 2005). Accordingly, AH are mostly described as visual pseudo-hallucinations (i.e. with preserved insight about the hallucinatory nature of the experience), differing further from HAS and OBE, for which insight is most often absent (Blanke et al., 2004, Blanke and Mohr, 2005). Previous work found that most of the present patients with AH had lesion overlap in the right superior occipital gyrus and the right cuneus in extrastriate visual cortex (Heydrich and Blanke, 2013). The present connectivity data confirm the importance of visual mechanisms in AH by revealing the involvement of extrastriate visual cortex (containing brain regions specialized for visual processing of body and face stimuli (Allison et al., 1994, Downing and Kanwisher, 2001), extending the lesion overlap data (Heydrich and Blanke, 2013). The present data extend these earlier lesion data by revealing additional altered connectivity with ventral stream regions (Ungerleider and Haxby, 1994) at the level of the pITG (located in proximity to the FFA), which has been associated with own face recognition (Allison et al., 1994). Another novel observation was our finding that AH lesions showed connectivity with the bilateral precuneus/superior parietal cortex, which is part of the dorsal stream (Ungerleider and Haxby, 1994) relevant for spatial perception and sensorimotor integration and planning (Ungerleider and Haxby, 1994, Conway, 2018). The precuneus is involved in many self-related functions, including own face perception, self-location, self-projection (Cavanna and Trimble, 2006, Northoff et al., 2006), and own-body processing (Platek et al., 2008), coherent with our finding of precuneus involvement only in AH patients, as they often report seeing their own face during the AP, sometimes as clearly as if they were looking in a mirror.
4.2. Heautoscopy (HAS)
We found that lesions causing HAS are part of a common brain network functionally connected to large bilateral STG/MTG clusters, but also to clusters in fronto-temporal cortex as well as subcortical regions. From these areas only three left hemispheric regions were found to be specifically linked to HAS: the left IFG, the left insula and the PHC. In HAS, compared to the other AP, patients experience a mental state that is characterized by a strong alteration of bodily self-consciousness with alternating self-location, visuo-spatial perspective and self-identification between the physical body and the autoscopic body (Brugger, 2002, Blanke et al., 2004, Heydrich and Blanke, 2013, Blanke and Mohr, 2005, Brugger et al., 1994). We found that HAS was associated with left IFG, which has been involved in several self-related processes (Morin and Michaud, 2007) such as face and body identification (Platek et al., 2008, Uddin et al., 2005) coherent with HAS phenomenology where the patients report abnormal and strong self-identification with the autoscopic body (Heydrich and Blanke, 2013). In addition, the left IFG is involved in inner speech and language processing (Morin and Michaud, 2007, McGuire et al., 1996), which is arguably in line with the report of thought and verbal communication with the autoscopic body. The left IFG and the left TPJ (the latter is affected commonly in all patients with AP; Fig. 4) are key cortical language regions. Both regions have also been involved in inner speech and language processing (Morin and Michaud, 2007, McGuire et al., 1996, Shergill et al., 2001, Geva et al., 2011, Liakakis et al., 2011, Hubl et al., 2004) and in auditory verbal hallucinations, based on functional connectivity work in patients with such hallucinations (Allen et al., 2008, Alderson-Day et al., 2016, Jardri et al., 2011). Recent hypotheses also highlighted the role of connectivity mechanisms between major brain networks such as the default mode network and the language network, which when impaired have been associated with hallucinations (Alderson-Day et al., 2016). Based on our observation of dysfunctional connectivity in HAS patients with the left IFG in the present study, the presence of language-related phenomenology (of the autoscopic body), as well as previous lesion data linking HAS to damage in the left hemisphere (Heydrich and Blanke, 2013), we speculate that the auditory-verbal phenomenology of HAS, as compared to OBE and AH, may partly result from disrupted functional connectivity, spread of epileptic activity, or release phenomena (Braun et al., 2003) with brain regions involved in language and/or auditory-verbal perceptual processes.
Another experiential feature, that distinguishes HAS from AH and OBE, is the experience of echopraxia with the autoscopic body (Brugger et al., 2006): disconnection from left IFG may reflect such echopraxia-related aspects of HAS, due to interference with sensorimotor processing, conscious action monitoring and action observation (Molnar-Szakacs et al., 2005, Nahab et al., 2011). The left insula found in the present data extends previous lesion overlap data (Heydrich and Blanke, 2013) and is in line with the insula being a multisensory brain region integrating somatosensory, motor, vestibular, visual and interoceptive signals (Flynn et al., 1999). Abnormal integration of these signals has been used in healthy subjects to experimentally induce changes in hand ownership (Tsakiris et al., 2007) as well as changes in self-identification (Ionta et al., 2014), compatible with the prominent alteration of self-identification in patients with HAS (Heydrich and Blanke, 2013, Brugger et al., 1994). We also found that HAS was associated with PHC, which has not been reported before. PHC is a key region for episodic memory, autobiographical memory, and spatial navigation (Ranganath and Ritchey, 2012, Aminoff et al., 2013), as well as viewpoint-specific local scene processing (Ranganath and Ritchey, 2012). Even though, the present data show that HAS is associated with bilateral networks and that lesions occurring in the right hemisphere (Heydrich and Blanke, 2013) can also cause HAS, only left functional connections at left IFG, left insula and left PHC were specific for HAS, highlighting the importance of the left hemisphere in HAS, coherent with frequent experiences of speech and thought communication with the autoscopic double in HAS.
4.3. Out-of-body experiences (OBE)
Lesions causing OBE are part of a common brain network functionally connected to bilateral angular gyrus, bilateral MTG and inferior temporal cortex, right precuneus, left cerebellum and several clusters in right prefrontal cortex, mostly IFG. Several of these regions were specific to OBE and included the bilateral angular gyrus, right IFG, right precuneus, right MTG. Compared to other AP, only OBE patients report seeing their own body from an elevated visuo-spatial perspective that is associated with prominent vestibular sensations (Blanke et al., 2004) and being located at this elevated position (i.e. abnormal self-location and disembodiment). The angular gyrus of TPJ is a multisensory area involved in visuo-tactile and vestibular processing (Ionta et al., 2011, Ventre-Dominey, 2014), in line with previous lesion overlap data (Ionta et al., 2011) and single case reports (Devinsky et al., 1989, Maillard et al., 2004, Brandt et al., 2005). Moreover, vestibular processing is thought to arise from a network of connected regions around the core regions of TPJ and parieto-insular vestibular cortex, including frontal and temporal regions such as IFG and ITG, also highlighted in our data (Ventre-Dominey, 2014). TPJ involvement (bilateral angular gyrus) in OBEs is also compatible with data in healthy subjects when experiencing experimentally-induced changes in self-location and elevated visuo-spatial perspective (Ionta et al., 2011); as well as perspective taking during mental rotation (Zeugin et al., 2020) and when mentalizing psychological closeness (Ionta et al., 2020). Altogether, this highlights the prominent involvement of the TPJ in self-consciousness more generally (Vogeley et al., 2004). The OBE network also included connectivity with right precuneus and IFG. PET imaging revealed recruitment of the precuneus in a single case of stimulation-induced OBE (De Ridder et al., 2007), likely reflecting this structure’s role in visual-spatial processing and in self-related processing (Cavanna and Trimble, 2006, Northoff et al., 2006) and perspective taking (Vogeley et al., 2004), which are disrupted in OBE. Concerning the connectivity with the IFG, only the right IFG was associated with OBE and we argue that this likely reflects the IFG’s role in self-processing and self-recognition (Uddin et al., 2005), with conflict monitoring, and self-other distinction (Nahab et al., 2011), as well perspective taking coherent with OBE phenomenology (Vogeley et al., 2004). Future work should investigate how these self-related processes in right vs left IFG are associated with OBE vs. HAS, respectively. Finally, we note that the present data did not allow us to investigate in more depth whether our patients’ neurological or neuropsychological deficits are associated with the present functional connectivity findings. AP based on focal brain damage are rare and the number of patients per each form of AP was too low and thus did not allow us to investigate these aspects in the present study (see Supplementary Materials for exploratory results for sensorimotor and visual deficits).
4.4. Common regions and conclusion
Our data show that lesions causing AP share connectivity with bilateral TPJ, in agreement with current classification of AP and the proposed idea of continuum between AP (Hécaen and Ajuriaguerra, 1952, Maillard et al., 2004) where all AP are associated with illusory reduplication of one’s own body, but resulting from disrupted functional connectivity with distinct functional sub-systems (Brugger, 2002, Blanke et al., 2004). Thus, each AP results from a different lesion location and different altered network connectivity (Fig. 2), but localize to a single common network at the bilateral TPJ (Fig. 4). We argue that the TPJ involvement reflects the main common element of AP – the illusory visual reduplication of one’s own body, which is further associated with specific patterns of altered functional connectivity that defines each AP: AH are visual AP, OBE have prominent vestibular and spatial components associated with disembodiment and perspective changes and HAS has prominent motor and language-related aspects. The present connectivity findings may also explain why patients may report more than one AP, as for example in the case of an AP of epileptic origin the involved network may differ depending on the spread of ictal activity in a given seizure (Maillard et al., 2004, Tadokoro et al., 2006, Arias et al., 2007). Smaller clusters of common connectivity among the three AP were also found and involved the bilateral hippocampus/ parahippocampus. We speculate that this involvement may reflect the key role of medial temporal cortex in episodic memory and autobiographical memory as well as spatial navigation (Clower et al., 2001) and self-location (Guterstam et al., 2015).
The combined origin of focal brain damage and altered network connectivity accounts well for the multiple medical causes of AP, which include various focal and generalized neurological diseases. AP are reported for many etiologies including focal epilepsy (Devinsky et al., 1989, Blanke et al., 2004, Maillard et al., 2004), vascular brain damage (Blanke et al., 2004), and arteriovenous malformationy (Devinsky et al., 1989) among others. The present data accounts for the wide variety of pathological brain mechanisms and provides a combined lesion- and connectivity-based framework that may also be or relevance for AP of generalized neurological etiology (i.e. generalized epilepsies Blanke et al., 2004).
Funding
This work was supported by the Bertarelli Foundation, the Swiss National Science Foundation (SNF) and two generous donors advised by CARIGEST SA, the first one wishing to remain anonymous and second one being Fondazione Teofilo Rossi di Montelera e di Premuda. E.B. is supported The National Center of Competence in Research (NCCR) “Synapsy – The Synaptic Bases of Mental Diseases” (# 51AU40-125759). L.H. is supported by the Swiss National Science Foundation (#33CM30-124089) and the Cogito Foundation.
CRediT authorship contribution statement
Eva Blondiaux: Conceptualization, Methodology, Formal analysis. Lukas Heydrich: Investigation, Writing - review & editing. Olaf Blanke: Conceptualization, Investigation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102612.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alderson-Day B., Diederen K., Fernyhough C. Auditory hallucinations and the Brain’s resting-state networks: findings and methodological observations. Schizophr. Bull. 2016;42:1110–1123. doi: 10.1093/schbul/sbw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P., Larøi F., McGuire P.K., Aleman A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Allison T., Ginter H., McCarthy G. Face recognition in human extrastriate cortex. J. Neurophysiol. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Moshe B. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzellotti F., Onofrj V., Maruotti V. Autoscopic phenomena: case report and review of literature. Behav Brain Funct [online serial]. 2011;7:2. doi: 10.1186/1744-9081-7-2. http://www.biomedcentral.com/content/pdf/1744-9081-7-2.pdf%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3032659&tool=pmcentrez&rendertype=abstract Accessed at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M., Constela I.R., Iglesias S., Arias-Rivas S., Dapena D., Sesar Á. The autoscopic phenomena in neurological clinic: a study of two cases. J Neurol Sci. 2007;263:223–225. doi: 10.1016/j.jns.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Blanke O., Castillo V. Clinical Neuroimaging in Epileptic Patients with Autoscopic Hallucinations and Out-of-Body Experiences. Epileptologie. 2007:90–96. [Google Scholar]
- Blanke O., Mohr C. Out-of-body experience, heautoscopy, and autoscopic hallucination of neurological origin: Implications for neurocognitive mechanisms of corporeal awareness and self-consciousness. Brain Res Rev. 2005;50:184–199. doi: 10.1016/j.brainresrev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Blanke O., Landis T., Spinelli L., Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain. 2004;127:243–258. doi: 10.1093/brain/awh040. [DOI] [PubMed] [Google Scholar]
- Blanke, O., 2012. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci [online serial]. Nature Publishing Group; 13, pp. 556–571. Accessed at: http://dx.doi.org/10.1038/nrn3292. Accessed July 9, 2014. [DOI] [PubMed]
- Boes A.D., Prasad S., Liu H. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N., Làdavas E., Farnè A. Spatial Perspective and Coordinate Systems in Autoscopy: A Case Report of a “ Fantome de Profil ” in Occipital Brain Damage. Epub. 2011:1741–1751. doi: 10.1162/jocn.2010.21569. [DOI] [PubMed] [Google Scholar]
- Brandt Brechtelsbauer D., Bien C.G., Reiners K. “Out-of-body experience” als mögliches anfallssymptom bei einem patienten mit rechtsparietaler läsion. Nervenarzt. 2005;76:1259–1262. doi: 10.1007/s00115-005-1904-y. [DOI] [PubMed] [Google Scholar]
- Braun C.M.J., Dumont M., Duval J., Hamel-hébert I., Godbout L. Lesions that cause visual, auditory or somatic hallucination. 2003;28:432–449. [PMC free article] [PubMed] [Google Scholar]
- Brugger P., Agosti R., Regard M., Wieser H.G., Landis T. Heautoscopy, epilepsy, and suicide. J Neurol Neurosurg Psychiatry [online serial]. 1994;57:838–839. doi: 10.1136/jnnp.57.7.838. http://jnnp.bmj.com/cgi/doi/10.1136/jnnp.57.7.838 Accessed at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger, Regard, M., Landis, T., 1997. Illusory reduplication of one’s own body: phenomenology and classification of autoscopic phenomena. Cogn. Neuropsychiatry [online serial] 2:19–38. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/25420137. [DOI] [PubMed]
- Brugger P., Regard M., Landis T., Oelz O. Hallucinatory experiences in extreme-altitude climbers. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999:67–71. [PubMed] [Google Scholar]
- Brugger P., Blanke O., Regard M., Bradford D.T., Landis T. Polyopic heautoscopy: Case report and review of the literature. Cortex. 2006;42:666–674. doi: 10.1016/s0010-9452(08)70403-9. [DOI] [PubMed] [Google Scholar]
- Brugger, 2002. Reflective mirrors: Perspective-taking in autoscopic phenomena. Cogn. Neuropsychiatry 7, 179–194. [DOI] [PubMed]
- Cavanna A.E., Trimble M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Clower D., West R.A., Lynch J.C., Strick P.L. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. [online serial]. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. http://www.ncbi.nlm.nih.gov/pubmed/11487651 Accessed at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B.R. The Organization and Operation of Inferior Temporal Cortex. Annu. Rev. Vis. Sci. [online serial]. 2018;4:381–402. doi: 10.1146/annurev-vision-091517-034202. https://www.annualreviews.org/doi/10.1146/annurev-vision-091517-034202 Accessed at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby R.R., Laganiere S., Pascual-Leone A., Prasad S., Fox M.D. Finding the imposter: Brain connectivity of lesions causing delusional misidentifications. Brain. 2017;140:497–507. doi: 10.1093/brain/aww288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Van Laere K., Dupont P., Menovsky T., Van de Heyning P. Visualizing out-of-body experience in the brain. N. Engl. J. Med. 2007;357:1829–1833. doi: 10.1056/NEJMoa070010. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Feldmann E., Burrowes K., Bromfield E. Autoscopic phenomena with seizures. Arch Neurol. 1989;46:1080–1088. doi: 10.1001/archneur.1989.00520460060015. [DOI] [PubMed] [Google Scholar]
- Downing P., Kanwisher N. A cortical area specialized for visual processing of the human body. J. Vis. 2001;1:2470–2474. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Flynn F.G., Benson D.F., Ardila A. Anatomy of the insula - Functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Geva S., Jones P.S., Crinion J.T., Price C.J., Baron J.C., Warburton E.A. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain. 2011;134:3071–3082. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterstam A., Björnsdotter M., Gentile G., Ehrsson H.H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. [online serial]. 2015;25:1416–1425. doi: 10.1016/j.cub.2015.03.059. http://linkinghub.elsevier.com/retrieve/pii/S0960982215004121 Accessed at: [DOI] [PubMed] [Google Scholar]
- Hécaen H., Ajuriaguerra J. Meconnaissances et Hallucinations Corporelles: Intégration et Désintégration de la Somatognosie. Masson (in French) 1952 [PubMed] [Google Scholar]
- Heydrich L., Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain. 2013;136:790–803. doi: 10.1093/brain/aws364. [DOI] [PubMed] [Google Scholar]
- Hubl D., Koenig T., Strik W. Pathways that make voices. Arch. Gen. Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Ionta S., Costantini M., Ferretti A., Galati G., Romani G.L., Aglioti S.M. Visual similarity and psychological closeness are neurally dissociable in the brain response to vicarious pain. Cortex [online serial]. Elsevier Ltd. 2020;133:295–308. doi: 10.1016/j.cortex.2020.09.028. [DOI] [PubMed] [Google Scholar]
- Ionta, S., Heydrich, L., Lenggenhager, B., et al., 2011. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron [online serial]. Elsevier Inc.; 70, 363–374. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/21521620. Accessed September 11, 2014. [DOI] [PubMed]
- Ionta, S., Martuzzi, R., Salomon, R., Blanke, O., 2014. The brain network reflecting bodily self-consciousness: a functional connectivity study. Soc Cogn Affect Neurosci [online serial]. Epub 2014 Jan 30. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/24396007. Accessed August 13, 2014. [DOI] [PMC free article] [PubMed]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. Diversity of the inferior frontal gyrus-A meta-analysis of neuroimaging studies. Behav. Brain Res. [online serial] Elsevier B.V. 2011;225:341–347. doi: 10.1016/j.bbr.2011.06.022. Accessed at: http://dx.doi.org/10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Maillard L., Vignal J.P., Anxionnat R., Taillandier L., Vespignani H. Semiologic Value of Ictal Autoscopy. Epilepsia. 2004;45:391–394. doi: 10.1111/j.0013-9580.2004.39103.x. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Silbersweig D.A., Murray R.M., David A.S., Frackowiak R.S.J., Frith C.D. Functional anatomy of inner speech and auditory verbal imagery. Psychol. Med. 1996;26:29–38. doi: 10.1017/s0033291700033699. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I., Iacoboni M., Koski L., Mazziotta J.C. Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cereb. Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Morin A., Michaud J. Self-awareness and the left inferior frontal gyrus: Inner speech use during self-related processing. Brain Res. Bull. 2007;74:387–396. doi: 10.1016/j.brainresbull.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Nahab F.B., Kundu P., Gallea C. The neural processes underlying self-agency. Cereb. Cortex. 2011;21:48–55. doi: 10.1093/cercor/bhq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner K.B., Colcombe S.J., Tobe R.H. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front Neurosci [online serial]. 2012;6:1–11. doi: 10.3389/fnins.2012.00152. http://journal.frontiersin.org/article/10.3389/fnins.2012.00152/abstract Accessed at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Platek S.M., Wathne K., Tierney N.G., Thomson J.W. Neural correlates of self-face recognition: An effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Power, J.D., Barnes, K.A., Snyder, A.Z., Schlaggar, B.L., Petersen, S.E., 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage [online serial]. Elsevier Inc.; 2012;59:2142–2154. Accessed at: http://dx.doi.org/10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed]
- Ranganath, C., Ritchey, M., 2012. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci [online serial]. Nature Publishing Group 13, pp. 713–726. Accessed at: http://dx.doi.org/10.1038/nrn3338. [DOI] [PubMed]
- Rorden C., Karnath H.-O., Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Bullmore E.T., Brammer M.J., Williams S.C.R., Murray R.M., McGuire P.K. A functional study of auditory verbal imagery. Psychol. Med. 2001;31:241–253. doi: 10.1017/s003329170100335x. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y., Oshima T., Kanemoto K. Postictal autoscopy in a patient with partial epilepsy. Epilepsy Behav. 2006;9:535–540. doi: 10.1016/j.yebeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Hesse M.D., Boy C., Haggard P., Fink G.R. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cereb. Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kaplan J.T., Molnar-Szakacs I., Zaidel E., Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: An event-related fMRI study. Neuroimage. 2005;25:926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G., Haxby J.V. “What” and “where” in the human brain. Curr. Opin. Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ventre-Dominey J. Vestibular function in the temporal and parietal cortex: Distinct velocity and inertial processing pathways. Front Integr. Neurosci. 2014;8:1–13. doi: 10.3389/fnint.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley May M, Ritzl A., Falkai P., Zilles K., Fink G.R. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Zamboni G., Budriese C., Nichelli P. “Seeing oneself”: A case of autoscopy. Neurocase. 2005;11:212–215. doi: 10.1080/13554790590944799. [DOI] [PubMed] [Google Scholar]
- Zeugin D., Notter M.P., Knebel J.F., Ionta S. Temporo-parietal contribution to the mental representations of self/other face. Brain Cogn. [online serial]. Elsevier. 2020;143:105600. doi: 10.1016/j.bandc.2020.105600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding authors upon request.