Table 4. Topological Parameters for Intermolecular Interactions (in Different Dimers of Compound 1) at Their (3, −1) BCPsa.
| interaction | Rij | ρ(r) | ∇2ρ(r) | V(r) | G(r) | H(r) | 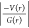 |
De |
|---|---|---|---|---|---|---|---|---|
| I | ||||||||
| C8–H8···C3A | 2.928 | 0.079 | 0.981 | –18.8 | 22.8 | 4.0 | 0.83 | 2.3 |
| S1A···C1 | 3.414 | 0.053 | 0.613 | –11.4 | 14.1 | 2.6 | 0.81 | 1.4 |
| II | ||||||||
| S2···Cl1 | 3.421 | 0.059 | 0.737 | –12.3 | 16.2 | 3.9 | 0.76 | 1.5 |
| III | ||||||||
| C6A–H6A···N2 | 2.362 | 0.079 | 1.163 | –20.0 | 25.8 | 5.9 | 0.77 | 2.4 |
| C6A–H6A···N1 | 2.451 | 0.073 | 1.167 | –18.8 | 25.3 | 6.5 | 0.74 | 2.3 |
| C5A–H5A···S1A | 3.021 | 0.035 | 0.408 | –6.1 | 8.6 | 2.5 | 0.71 | 0.7 |
| IV | ||||||||
| C19–H19···S1A | 2.818 | 0.060 | 0.645 | –12.0 | 14.8 | 2.8 | 0.81 | 1.4 |
| C13–H13B···C6A | 2.810 | 0.042 | 0.437 | –7.4 | 9.7 | 2.2 | 0.77 | 0.9 |
| V | ||||||||
| F1···C10(π) | 3.839 | 0.019 | 0.261 | –3.9 | 5.5 | 1.6 | 0.70 | 0.5 |
| VI | ||||||||
| C9–H9···F1 | 2.249 | 0.068 | 1.149 | –19.1 | 25.2 | 6.1 | 0.76 | 2.3 |
Definitions: Rij, bond path (Å); ρ(r), electron density (e Å–3); ∇2ρ(r), Laplacian of electron density (e Å–5); V(r), potential electron density (kJ mol–1 br–3); G(r), kinetic electron density (kJ mol–1 br–3); H(r), total electronic energy density (kJ mol–1 br–3); De, dissociation energy (kcal mol–1).
