Abstract
PURPOSE:
The purpose of this study was to evaluate whether papilledema severity is associated with specific demographic or clinical factors in patients with idiopathic intracranial hypertension (IIH).
MATERIALS AND METHODS:
A retrospective cohort study of consecutive IIH patients seen at one tertiary care institution between 1989 and March 31, 2017 was performed. IIH patients were classified as mild (Frisén Grade 1 or 2) or severe (Frisén Grade 4 or 5) based on grading of fundus photographs obtained at first presentation. Demographic and clinical variables including age, body mass index (BMI), gender, visual acuity, Humphrey visual field mean deviation, and cerebrospinal fluid (CSF) opening pressure were extracted from patient medical records for statistical analyses.
RESULTS:
A total of 239 patients were included in the study: 152 with mild papilledema and 87 with severe papilledema. There was no difference in age, race, BMI, or male gender between the mild and severe papilledema groups. CSF opening pressure was significantly higher in the severe papilledema group (41.89 cm of water vs. 33.69, 95% confidence interval [CI]: −10.79–−5.62, P < 0.0001). There was a significant difference in the Humphrey mean deviation (−6.38 dB compared to − 3.25 dB, 95% CI: −4.82–−1.44 dB, P < 0.001) and average logarithm of the minimum angle of resolution visual acuity at final follow-up (0.21 vs. 0.045, 95% CI: −0.299–−0.040 , P = 0.01).
CONCLUSION:
Age, race, sex, and BMI were similar in IIH patients with mild versus severe papilledema, emphasizing the importance of a dilated fundus examination to reliably stratify patients. Patients with severe papilledema are at higher risk of visual acuity and visual field loss at final follow-up.
Keywords: Demographics, idiopathic intracranial hypertension, papilledema, visual outcome
Introduction
Idiopathic intracranial hypertension (IIH) is a condition seen primarily in young, obese women and characterized by symptoms and signs of raised intracranial pressure without an identifiable cause.[1] Patients with IIH are at risk for severe and irreversible vision loss, and regular neuro-ophthalmological monitoring is required to ensure that timely medical or surgical treatment is initiated. Irreversible vision loss has been found to be more common in IIH patients who are male,[2] of black race,[3] and those with coexisting hypertension,[4] anemia,[5] and morbid obesity.[6] A poor visual outcome was also associated with the severity of papilledema at presentation in large retrospective studies and in the prospective Idiopathic Intracranial Hypertension Treatment Trial (IIHTT).[7,8] The ability to stratify patients into risk categories helps in triaging, clinical decision-making, and identifying at-risk population groups who may require more frequent monitoring and aggressive interventions. The goal of this study was to determine whether the severity of papilledema at presentation is associated with specific demographic or clinical factors in IIH patients.
Methods
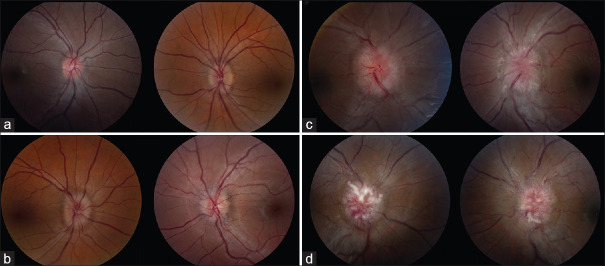
This study was conducted in compliance with the US Health Insurance Portability and Accountability Act of 1996 and received research ethics board approval from our institution (approval number: 89411), and the patient consent was waived by IRB. Consecutive IIH patients who underwent neuro-ophthalmological evaluation at one tertiary care academic center between 1989 and March 31, 2017 were reviewed for inclusion in the study. Only patients with fundus photographs at presentation were included. The fundus photographs must have been taken before or within 30 days of diagnostic lumbar puncture or treatment with acetazolamide. Those with optic atrophy or individuals who underwent surgical treatment before photographs were taken were excluded. Fundus photographs must have been of sufficient quality and were graded by three independent ophthalmologists in a standardized fashion as previously described[8] according to the modified Frisén scale.[9] Patients with Frisén Grades 1–2 were included in the mild papilledema group and those with Frisén Grades 4–5 were included in the severe papilledema group [Figure 1]. Patients with Frisén Grade 3 papilledema were excluded. Assignment to group was determined by the eye with the most severe optic disc edema.
Figure 1.
Fundus photographs demonstrating mild and severe papilledema. Mild papilledema was defined as Frisén Grade 1 (a), which is a C-shaped blurred nasal optic disc margin and Frisén Grade 2 (b), which is circumferential optic disc edema. Severe papilledema was defined as Frisén Grade 4(c), which has obscuration of a major vessel on the optic disc and Frisén Grade 5(d), which has total obscuration of all the major vessels on the disc
Demographic information and clinical variables were extracted for statistical analyses. These variables were routinely obtained during the neuro-ophthalmological evaluation and included age, gender, body mass index (BMI), race, cerebrospinal fluid (CSF) opening pressure, visual acuity, and visual field mean deviation. Snellen visual acuity was converted to logarithm of the minimum angle of resolution (logMAR) for analysis. These variables were statistically correlated to the presence of mild or severe papilledema. Statistical analysis was performed using R: a language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). Variables of interest were compared between the mild and severe papilledema groups using mean and standard deviations for continuous variables and frequencies and percentages for categorical variables. Comparisons between the mild and severe papilledema groups were performed using two-sample t-tests for continuous variables and Chi-square tests for categorical variables. The mean logMAR visual acuity values were compared between the mild and severe groups at final follow-up using a Welch two-sample t-test. All statistical tests were completed using an alpha of 0.05.
Results
A total of 239 patients were included in the study: 152 with mild papilledema and 87 with severe papilledema. In the mild papilledema group, 66 patients (43%) had Frisén Grade 1 papilledema and 86 patients (57%) had Frisén Grade 2 papilledema. In the severe papilledema group, 73 patients (84%) had Frisén Grade 4 papilledema and 14 patients (16%) had Frisén Grade 5 papilledema. Median age at diagnosis was 30.0 years (interquartile range: 24.7–36.0), 18 patients (7.5%) were men, and 118 (49.1%) were of black race. BMI at presentation was a mean of 38.2 kg/m2(standard deviation: 9.4) and CSF opening pressure was on average 37.0 cm of water (standard deviation: 9.5). Baseline logMAR visual acuity was 0.04 (standard deviation: 0.156) in the mild papilledema group and 0.25 (standard deviation: 0.50) in the severe papilledema group. Baseline Humphrey visual field mean deviation was − 5.42 dB (standard deviation: −5.97 dB). Final logMAR visual acuity was 0.04 (standard deviation: 0.18) in the mild papilledema group and 0.21 (standard deviation: 0.56) in the severe papilledema group. Final Humphrey mean deviation was −3.53 dB (standard deviation: −4.52 dB). Follow-up time was a median of 44.0 weeks (interquartile range: 15.0–101.4).
The demographic and clinical characteristics of the mild and severe papilledema groups are summarized in Table 1. There was no difference in age, race, BMI, or male gender between the mild and severe papilledema groups. CSF opening pressure was significantly higher in the severe papilledema group (41.89 compared to 33.69 cm of water, 95% confidence interval (CI): −10.79–−5.62, P < 0.0001). There was also a significant difference in the Humphrey visual field mean deviation (−6.38 dB compared to − 3.25 dB, 95% CI: −4.82–−1.44 dB, P < 0.001) and average logMAR visual acuity at final follow-up (0.21 vs. 0.045, 95% CI: −0.299–−0.040, P = 0.01).
Table 1.
Composite data (mean values) from patients with mild and severe papilledema
| Mild papilledema (n=152) | Severe papilledema (n=87) | 95% CI | P | |
|---|---|---|---|---|
| Age | 31.53 | 31.02 | −2.14-3.16 | 0.71 |
| Black race (%) | 47 | 55 | N/A | 0.36 |
| BMI | 38.64 | 37.24 | −1.35-4.15 | 0.31 |
| Male gender (%) | 8 | 7 | N/A | 0.77 |
| CSF-OP | 33.69 | 41.89 | −10.79-−5.62 | <0.001 |
| HVF-MD, final follow-up | −3.25 | −6.38 | −4.82-−1.44 | <0.001 |
| Average logMAR VA, final follow-up | 0.045 | 0.21 | −0.299-−0.040 | 0.01 |
BMI=Body mass index, CSF-OP=Cerebrospinal fluid opening pressure, HVF-MD=Humphrey visual field mean deviation, VA=Visual acuity, CI=Confidence interval, LogMAR=Logarithm of the minimum angle of resolution
Discussion
In our large cohort of IIH patients with mild and severe papilledema, there was no difference in age, race, gender, or BMI, but patients with severe papilledema had significantly higher CSF opening pressure. Patients with severe papilledema from IIH also had a significantly worse mean deviation on Humphrey visual field testing and significantly worse visual acuity at their last follow-up as compared to the mild papilledema group. These findings reinforce the importance of examination of the ocular fundus to identify the severity of papilledema in patients who present with signs and symptoms concerning for IIH since these patients cannot be reliably differentiated based solely on their demographic features. The severity of papilledema allows providers to better understand their patients' risk of vision loss, allowing for more aggressive treatment and follow-up to be initiated.
Our findings are similar to those obtained in the IIHTT in that age, gender, and BMI were not significantly different between treatment failure and nontreatment failure groups.[7] An additional retrospective study also found no difference between weight alone in IIH patients with no visual dysfunction, definite visual dysfunction, and severe visual loss.[10] However, a previous study examining IIH patients categorized by BMI found that those with a BMI of 40 kg/m2or greater were more likely to have severe papilledema at baseline.[6] There was no association between age and gender in that study. In a prospective study of 50 IIH patients, marked weight gain was found to be associated with a poor visual outcome.[11] Moreover, male gender has also been correlated with more than two times the risk of severe vision loss compared to women with IIH.[2] Although these demographic factors, primarily gender and BMI, have been found consistently to be associated with a worse final visual outcome, our current study did not find an association of these demographic features with initial severity of papilledema, suggesting that a detailed funduscopic examination is still required to better stratify patients.
Indeed, the severity of papilledema is the most well-established risk factor for a poor visual outcome in IIH. This has been confirmed in the IIHTT, which was a prospective study in patients with mild vision loss,[7] in retrospective studies,[8,10,12] and in pediatric patients.[13,14] The IIHTT also found an association between CSF opening pressure and Frisén grade of papilledema, although this was a weak association.[15] This is consistent with the presumed pathophysiology of papilledema in IIH, a process in which axoplasmic flow is interrupted as a result of mechanical or ischemic factors.[16] With more severe stasis of axoplasmic flow, the papilledema appears more severe and optic nerve dysfunction becomes more likely.[16] Another way of quantifying the degree of papilledema is with optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL); the degree of abnormal thickening of the RNFL has been shown to correlate well with the Frisén grade of papilledema and decreases with treatment.[17,18] Although OCT can, therefore, serve as a method to objectively quantify the severity of papilledema, it must be recognized that optic atrophy may develop in severe cases, resulting in a reduction in the RNFL thickness in the setting of ongoing vision loss more consistent with progression of poorly controlled disease than improvement of papilledema.
The strengths of our study include a relatively large sample size of IIH patients who are representative of the spectrum of disease seen in clinical practice. Unlike the IIHTT which included only patients with mild visual impairment, our cohort was comprised of patients with all degrees of vision loss. High-quality fundus photographs are obtained routinely on all patients at our center. A limitation of this study is the inherent deficiencies of the Frisén scale when assessing the severity of papilledema, especially on photographs, since the scale relies on visible changes, such as obscuration of a blood vessel leaving the optic disc, that are open to interpretation.[19,20] However, by specifically excluding patients with Frisén Grade 3 papilledema, we tried to avoid any debate regarding the classification of the disc appearance as mild or severe papilledema.
Conclusion
Age, race, sex, and BMI were similar in patients with mild versus severe papilledema in IIH, but patients with severe papilledema were at higher risk of vision loss, reconfirming the importance of examination of the ocular fundus to reliably stratify IIH patients at presentation. Characterization of papilledema could be made possible routinely in emergency and neurology settings by the use of digital fundus photography,[21] potentially coupled with artificial intelligence.[22]
Financial support and sponsorship
This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, USA, and by NIH/NEI core grant P30-EY006360 (Department of Ophthalmology).
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
Acknowledgments
The authors would like to acknowledge Laura Ward for her assistance with the statistical analysis of data.
References
- 1.Thurtell MJ. Idiopathic intracranial hypertension. Continuum (Minneap Minn) 2019;25:1289–309. doi: 10.1212/CON.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 2.Bruce BB, Kedar S, van Stavern GP, Monaghan D, Acierno MD, Braswell RA, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–9. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–7. doi: 10.1212/01.wnl.0000304746.92913.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri.Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–74. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 5.Biousse V, Rucker JC, Vignal C, Crassard I, Katz BJ, Newman NJ. Anemia and papilledema. Am J Ophthalmol. 2003;135:437–46. doi: 10.1016/s0002-9394(02)02062-7. [DOI] [PubMed] [Google Scholar]
- 6.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: Relation between obesity and visual outcomes. J Neuroophthalmol. 2013;33:4–8. doi: 10.1097/WNO.0b013e31823f852d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall M, Falardeau J, Fletcher WA, Granadier RJ, Lam BL, Longmuir RA, et al. Risk factors for poor visual outcome in patients with idiopathic intracranial hypertension. Neurology. 2015;85:799–805. doi: 10.1212/WNL.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micieli JA, Bruce BB, Vasseneix C, Blanch RJ, Berezovsky DE, Peragallo JH, et al. Optic nerve appearance as a predictor of visual outcome in patients with idiopathic intracranial hypertension. Br J Ophthalmol. 2019;103:1429–35. doi: 10.1136/bjophthalmol-2018-313329. [DOI] [PubMed] [Google Scholar]
- 9.Scott CJ, Kardon RH, Lee AG, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–11. doi: 10.1001/archophthalmol.2010.94. [DOI] [PubMed] [Google Scholar]
- 10.Orcutt JC, Page NG, Sanders MD. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology. 1984;91:1303–12. doi: 10.1016/s0161-6420(84)34149-5. [DOI] [PubMed] [Google Scholar]
- 11.Wall M, George D. Idiopathic intracranial hypertension.A prospective study of 50 patients. Brain. 1991;114(Pt 1A):155–80. [PubMed] [Google Scholar]
- 12.Wall M, White WN., 2nd Asymmetric papilledema in idiopathic intracranial hypertension: Prospective interocular comparison of sensory visual function. Invest Ophthalmol Vis Sci. 1998;39:134–42. [PubMed] [Google Scholar]
- 13.Gospe SM, Bhatti MT, El-Dairi MA. Anatomic and visual function outcomes in paediatric idiopathic intracranial hypertension. Br J Ophthalmol. 2016;100:505–9. doi: 10.1136/bjophthalmol-2015-307043. [DOI] [PubMed] [Google Scholar]
- 14.Micieli JA, Bruce BB, Vasseneix C, Blanch RJ, Berezovsky DE, Newman NJ, et al. Influence of optic nerve appearance on visual outcome in pediatric idiopathic intracranial hypertension. Can J Neurol Sci. 2020 May;4:1–5. doi: 10.1017/cjn.2020.89. doi: 10.1017/cjn.2020.89. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Fischer WS, Wall M, McDermott MP, Kupersmith MJ, Feldon SE. NORDIC Idiopathic Intracranial Hypertension Study Group. Photographic reading center of the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT): Methods and baseline results. Invest Ophthalmol Vis Sci. 2015;56:3292–303. doi: 10.1167/iovs.15-16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trobe JD. Papilledema: The vexing issues. J Neuroophthalmol. 2011;31:175–86. doi: 10.1097/WNO.0b013e31821a8b0b. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja S, Anand D, Dutta TK, Roopesh Kumar VR, Kar SS. Retinal nerve fiber layer thickness analysis in cases of papilledema using optical coherence tomography – A case control study. Clin Neurol Neurosurg. 2015;136:95–9. doi: 10.1016/j.clineuro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Optical Coherence Tomography Substudy Committee, NORDIC Idiopathic Intracranial Hypertension Study Group. Papilledema outcomes from the optical coherence tomography substudy of the idiopathic intracranial hypertension treatment trial. Ophthalmology. 2015;122:1939–4500. doi: 10.1016/j.ophtha.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinclair AJ, Burdon MA, Nightingale PG, Matthews TD, Jacks A, Lawden M, et al. Rating papilloedema: An evaluation of the Frisén classification in idiopathic intracranial hypertension. J Neurol. 2012;259:1406–12. doi: 10.1007/s00415-011-6365-6. [DOI] [PubMed] [Google Scholar]
- 20.Sheils CR, Fischer WS, Hollar RA, Blanchard LM, Feldon SE NORDIC Idiopathic Intracranial Hypertension Study Group. The relationship between optic disc volume, area, and frisén score in patients with idiopathic intracranial hypertension. Am J Ophthalmol. 2018;195:101–9. doi: 10.1016/j.ajo.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biousse V, Bruce BB, Newman NJ. Ophthalmoscopy in the 21st century: The 2017 H.Houston Merritt Lecture. Neurology. 2018;90:167–75. doi: 10.1212/WNL.0000000000004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milea D, Najjar RP, Zhubo J, Ting D, Vasseneix C, Xu X, et al. Artificial intelligence to detect papilledema from ocular fundus photographs. N Engl J Med. 2020;382:1687–95. doi: 10.1056/NEJMoa1917130. [DOI] [PubMed] [Google Scholar]