Figure 5. Coarse-grained simulations of Aβ-nanoparticle interactions.
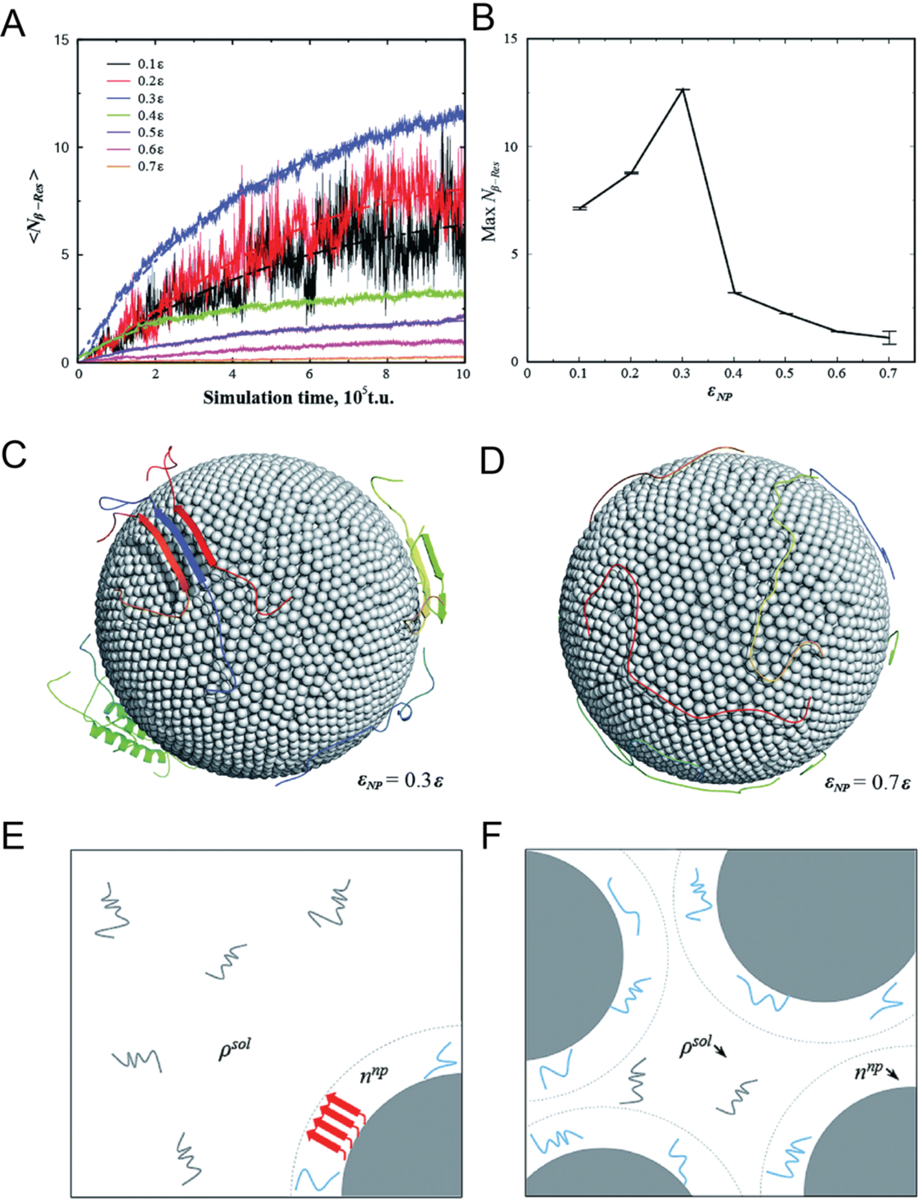
Dependence of Aβ aggregation on nanoparticle–protein interaction strength. (A) Average number of residues per chain that formed inter-peptide beta-sheets, Nβ–Res as a function of simulation time. (B) Maximum Nβ–Res as a function of nanoparticle-protein interaction strength εNP. Representative conformations with (C) εNP = 0.3ε (D) εNP = 0.74ε, where ε is the protein contact energy. (E) With a high protein/nanoparticle ratio, the nanoparticles (depicted as gray solid spheres) may promote the formation of amyloid fibrils (represented by red arrows) by increasing the local concentration of Aβ peptides on the nanoparticle surface (denoted as lines in cyan). (F) With a low protein/nanoparticle ratio, the nanoparticles displayed an inhibitive effect on the Aβ aggregation by depleting the peptides in solution but effectively having a low peptides concentration on nanoparticle surface. Reproduced with permission from Ref. 146. Copyrights 2015 The Royal Society of Chemistry.
