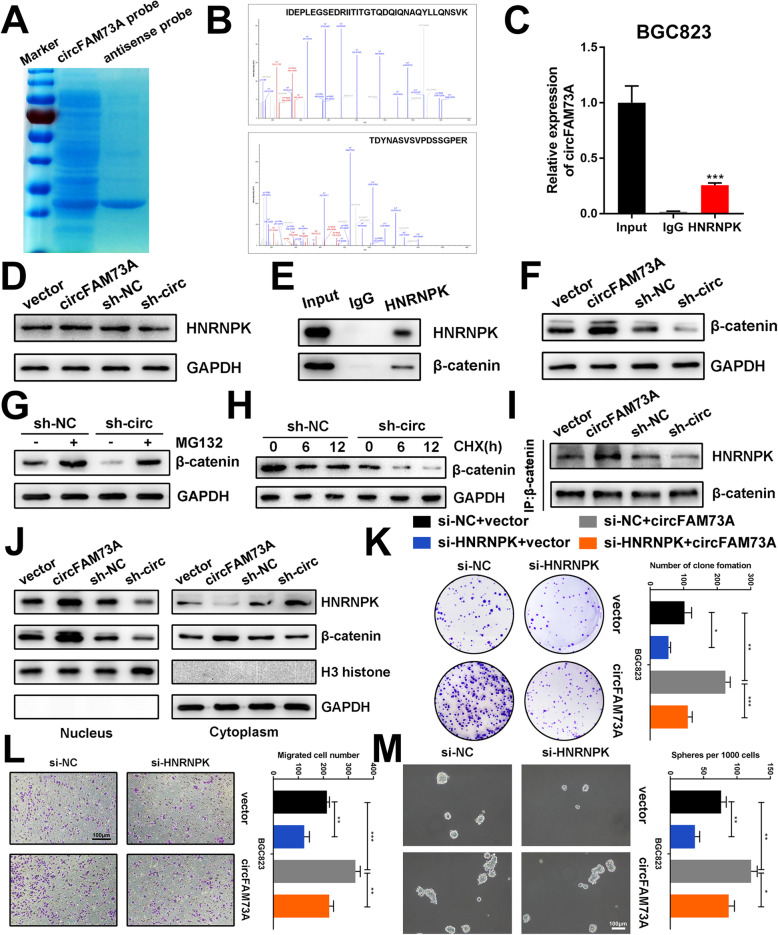
Fig. 9.
CircFAM73A interacts with HNRNPK and facilitates β-catenin stabilization. a Coomassie blue staining of circFAM73A pulldown. b Spectra of HNRNPK identified by mass spectrometry. c Relative abundance of circFAM73A detected by qRT-PCR after RIP using HNRNPK antibody in BGC823. d Expression of HNRNPK protein of BGC823 after circFAM73A overexpression or knocking-down measured by Western Blot. e Co-IP analysis using HNRNPK protein revealing the endogenous interaction between HNRNPK and β-catenin in BGC823. f Expression of β-catenin protein in BGC823 after circFAM73A overexpression or knocking-down measured by Western Blot. g Expression of β-catenin protein in BGC823 transfected with control shRNA or sh-circFAM73A and treated with MG132 (10 μmol/L, 10 h) or untreated measured by Western Blot. h Expression of β-catenin protein in BGC823 transfected with control shRNA or sh-circFAM73A and treated with cycloheximide (CHX, 50 μg/mL) for different time measured by Western Blot. i Co-IP and Western blot showing the interaction between HNRNPK and β-catenin after circFAM73A overexpression or knocking-down in BGC823. j The nuclear and cytoplasmic expression of HNRNPK and β-catenin measured by Western Blot after circFAM73A overexpression or knocking-down in BGC823. k-m Representative images of and quantification of clone formation (k) migrated cells (l) and formatted spheres (m) in BGC823 transfected with vector or circFAM73A plasmid and co-transfected with control siRNA or si-HNRNPK. Graph represents mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001