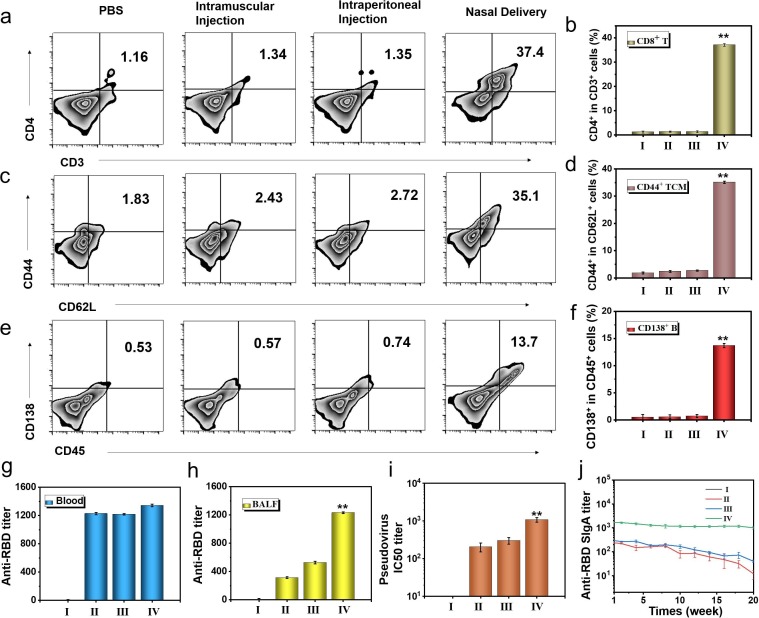
Fig. 8.
Immune protection of different administration routes of bionic-virus nanovaccine. (a) Representative flow cytometric analysis images of CD4+CD3+ T cells in BALF. (b) Relative quantification of CD4+CD3+ T cells in BALF. (c) Representative flow cytometric analysis images of CD44+CD62L+ TCM cells in BALF. (d) Relative quantification of CD44+CD62L+ TCM cells in BALF. (e) Representative flow cytometric analysis images of CD138+CD45+ B cells in spleen. (f) Relative quantification of CD138+CD45+ B cells in spleen. (g) Anti-RBD IgG titer. (h) Anti-RBD sIgA titer. (i) PsV IC50 inhibition titer of BALF. (j) Anti-RBD sIgA titer of mice during the five months evaluation period. (I: PBS, II: Intramuscular Injection, III: Intraperitoneal Injection, IV: Nasal Delivery).