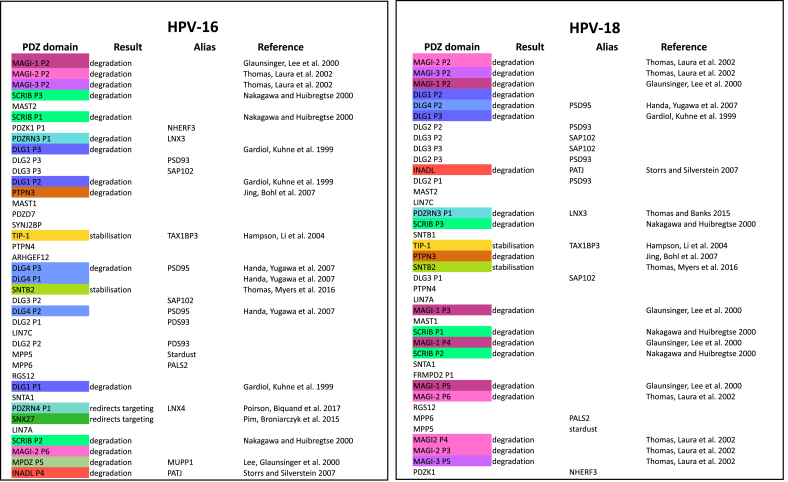
Table 1.
HPV-16 and HPV-18 E6s bind the same target proteins with different affinities and through different PDZ domains.
It has been shown in a number of studies that the E6 oncoproteins of different HPV types differ in their binding affinities for their various cellular target proteins [4,227,284]. This was demonstrated particularly clearly by Ref. [4]; who measured the binding affinities of HPV-16 and HPV-18 E6 for a huge array of different PDZ domains and in Table 1 we show the most strongly-bound PDZ domains of the two E6s in order, starting with the highest affinity, and with the previously confirmed interacting proteins defined in different colours.
It is immediately clear that the order of the most-favoured binding partners differs between the two E6s, although the second PDZ domains of the MAGI protein family members are confirmed as the highest binders of both [62,63,285], while SCRIB and DLG1 are, respectively, the second-favoured proteins of HPV-16 and HPV-18, as would be expected from previous results [226] and that HPV-16, but not HPV-18, binds to PDZRN4 [5].
Interestingly, however, the specific PDZ domain of a given protein also seems to differ: DLG1 binding was initially determined using HPV-18 and PDZ2 was defined as the binding domain, however HPV-16 appears to favour PDZ3; given the sequence differences between the two PBMs (16E6 is -SSRTRRETQL; 18E6 is -RLQRRRETQV) this may reflect the binding capacity of each PDZ domain for either a Leu (16E6) or Val (18E6) as the final residue, and there may well be a contribution from the upstream serines to 16E6 binding [229,286]. Thus, although the high-risk E6 proteins target a common set of PDZ-containing cellular proteins, it is clear that they may not all target them through the same PDZ domain and thus may compete with the binding of different cellular PDZ-binding proteins, with potentially differing secondary downstream effects within the infected cell.
It is also clear from this list that although many of these cellular proteins have been previously defined as E6 targets [[5], [59], [118], [119], [227], [287], [288], [290], [291], [292], [294], [295]], nearly half of the PDZ domains bound in this assay are from proteins that have not been so defined, and it might well be worth examining more closely their potential as targets, under various cellular conditions such as differentiation, hypoxia, or low nutrients.