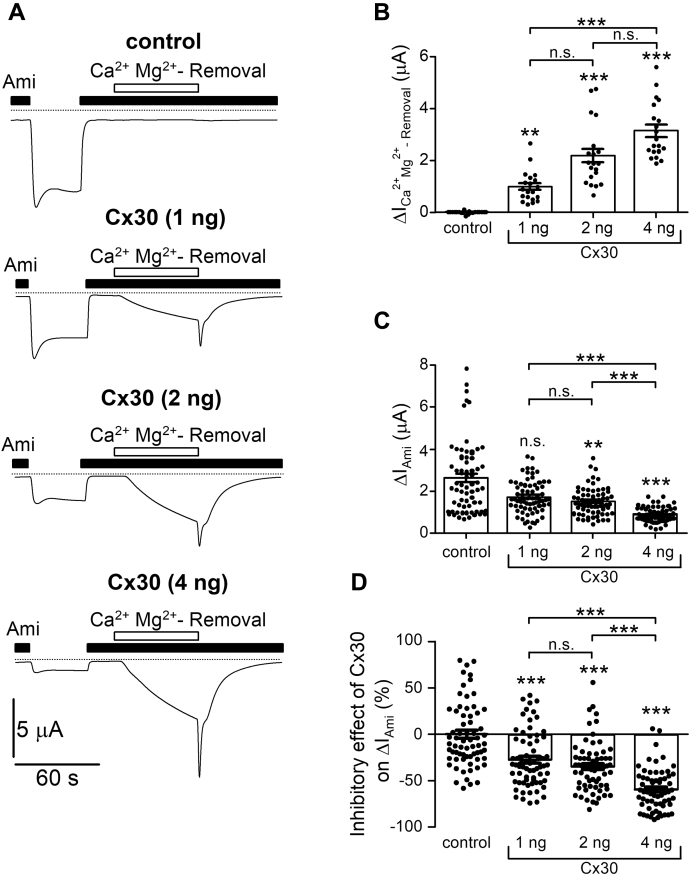
Figure 3.
Coexpression of Cx30 inhibits αβγENaC currents in a dose-dependent manner.A, representative whole-cell current traces recorded in a human αβγENaC-expressing control oocyte or in oocytes injected with both αβγENaC and different amounts of human Cx30 cRNA (1–4 ng/oocyte). Application of amiloride (Ami, 2 μM) and removal of divalent cations from the bath solution for 60 s (Ca2+Mg2+- Removal) are indicated by corresponding filled and open bars, respectively. Dashed lines indicate zero current level. B, ΔICa2+Mg2+-Removal values were obtained from similar experiments as shown in A and calculated as described in Figure 1. Mean ± SEM and individual data points for each experiment are shown (n = 20, N = 5). C, ENaC-mediated amiloride-sensitive current values (ΔIAmi) were calculated from similar experiments as shown in A by subtracting the baseline current in the presence of amiloride from the current level reached after amiloride washout. Mean ± SEM and data points for individual oocytes are shown; (n = 68, N = 5). D, the relative inhibitory effect of Cx30 on ENaC was calculated according to the following equation: where is the amiloride sensitive current of an individual oocyte coexpressing Cx30 and ENaC, whereas is the mean measured in control oocytes from the same batch but expressing ENaC alone (control). Original data are the same as in C. Mean ± SEM and data points for individual oocytes are shown; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant; compared with control (markers above the columns) or to another comparison group as indicated; Kruskall–Wallis with Dunn’s post hoc test.