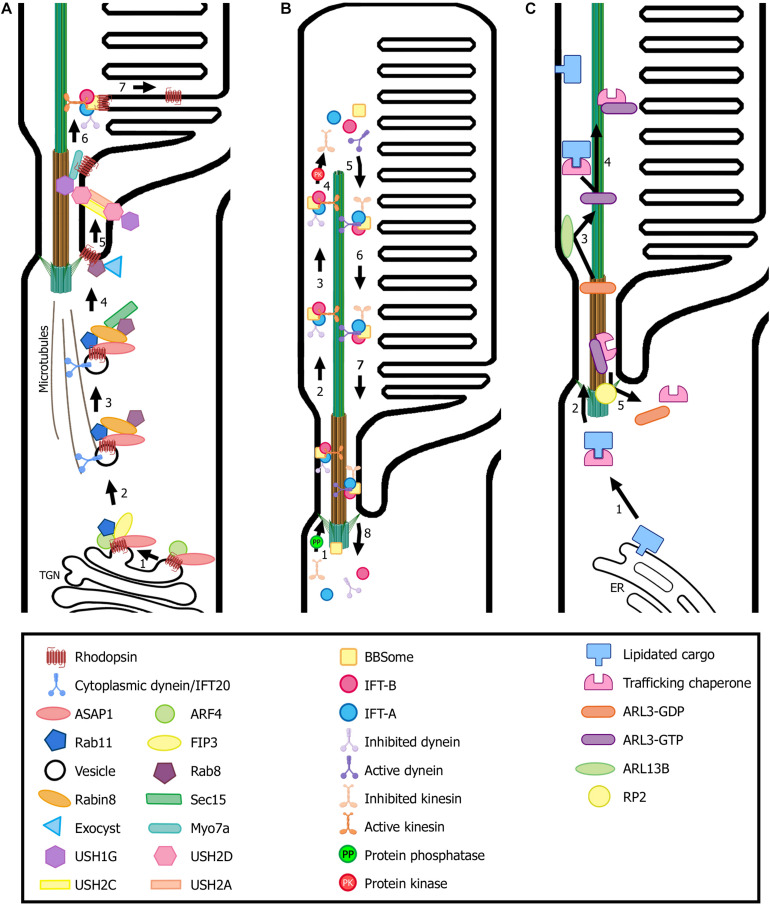
FIGURE 3.
Transport of ciliary proteins in photoreceptor cells. (A) Rhodopsin is transported from the trans-Golgi network (TGN) to the rod outer segment by a conventional secretory pathway regulated by small GTPases. Many ciliary protein complexes are involved in this process, performing different roles in trafficking that enable rhodopsin docking and transport through the connecting cilium to the ciliary membrane. Note the location of the USH protein complex formed by USH1G, USH2D, USH2A, and USH2C subunits. (B) The proposed model for the cycle of the IFT complexes in photoreceptors is based on flagella and primary cilium as well as cryo-EM structural models (Liang et al., 2014; Roberts, 2018; Toropova et al., 2019). IFT-B trains (responsible for anterograde trafficking) are assembled at the base of the cilium and move along the outer segment axoneme to the tip, where they are disassembled. Then, IFT-A trains are assembled at the tip and enable retrograde trafficking until they reach the base of the cilium, where they are disassembled to begin another trafficking cycle. Numbers in each panel indicate the sequence of transport steps. In all images, only selected proteins are represented, for the sake of clarity. (C) Transport and delivery of lipidated proteins from the endoplasmic reticulum (ER) to the outer segment is assisted by trafficking chaperones (PDE6D and UNC119b) and regulated by small GTPases (e.g., ARL3). (Figure created with BioRender.com).