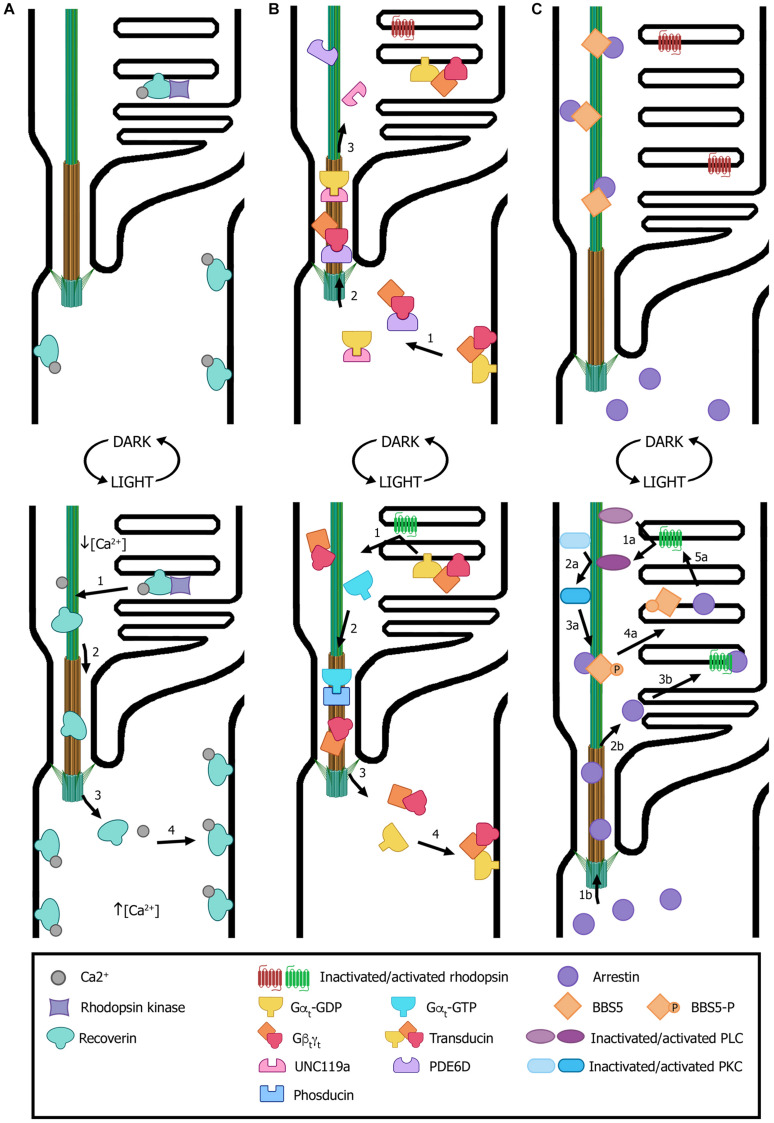
FIGURE 4.
Light-dependent translocation of signaling proteins. (A) In dark-adapted rods (top panel), recoverin is bound to Ca2+ and distributed along all subcellular compartments; in the outer segment, recoverin sequesters rhodopsin kinase. After light exposure (bottom panel), Ca2+ levels decrease in the outer segment and recoverin modifies its conformation to a Ca2+-free soluble form. This results in recoverin dissociation from disk membranes and its diffusion from the outer segment through the connecting cilium to other cellular compartments with higher Ca2+ concentrations, where it binds the cytoplasmic membrane. (B) In dark conditions (top panel), transducin subunits Gαt-GDP and Gβtγt are transported by the trafficking chaperones UNC119a and PDE6D, respectively, to the outer segment. There, the heterotrimer transducin binds to disk membranes. In light conditions (bottom panel), photoexcited rhodopsin induces GPD/GTP exchange in the Gαt subunit, resulting in heterotrimer dissociation and diffusion to the inner segment. Gαt translocation is assisted by phosducin. Once in the inner segment, GTP is hydrolyzed, causing Gαt-GdP and Gβtγt re-association and binding to the cytoplasmic membrane. (C) In dark conditions (top panel), arrestin is mostly found at the inner segment and the small fraction contained within the outer segment is sequestered by BBS5. Upon light exposure (bottom panel), activated arrestin can follow two independent pathways to interact with activated rhodopsin (pathways “a” and “b”). One pathway involves photoexcited rhodopsin interacting and activating phospholipase (PLC), which in turn activates protein kinase C (PKC). Activated PKC then phosphorylates BBS5, resulting in the release of arrestin, which can now diffuse freely and interact with activated rhodopsin (“1a” to “5a” steps). Alternatively, arrestin diffuses from the inner segment into the outer segment and binds to photoexcited rhodopsin (“1b” to “3b” steps). Numbers in each panel indicate the sequence of transport steps. In all images, only selected proteins are represented, for the sake of clarity. (Figure created with BioRender.com).