Abstract
Summary: Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) rarely affects the intracranial region without involvement of other sites. We report the case of a 68-year-old woman with isolated Rosai-Dorfman disease of the frontal dura. She presented with a new onset seizure. Initial MR imaging showed subtle mild change in the left frontal region. During the ensuing 8 months, a dural mass made its symptomatic and definite MR imaging appearance in the same region. No extracranial lesion was present.
Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) was first described in 1969 as a benign proliferative lesion with systemic symptoms and lymphadenopathy (1). It occurs more often in children and young adults and shows a mild male sex predilection (2). Rosai-Dorfman disease commonly involves cervical lymph nodes. Extranodal involvement is well recognized and includes several organs, including respiratory tract, skin, nasal cavity, orbit, and bone (2). Isolated intracranial Rosai-Dorfman disease is extremely rare (3–8). We report a case of isolated intracranial Rosai-Dorfman disease occurring in a relatively older patient, which was shown by histologic examination to have dura-based subarachnoidal involvement.
Case Report
A 68-year-old woman presented at another clinic with a new onset psychomotor seizure in October 2000. Her familial history was noncontributory. MR imaging of the head showed an effacement of the sulci and mild thickening of the cortex at the left frontal region (Fig 1). No definite lesion was found at the dura. Contrast-enhanced MR imaging was not performed at that time. The results of EEG were unremarkable. The same was true of laboratory tests, including basic hematologic parameters. The patient came to feel well during a period of 2 weeks. No additional examination was performed. She underwent follow-up examination at another clinic and did not receive therapy.
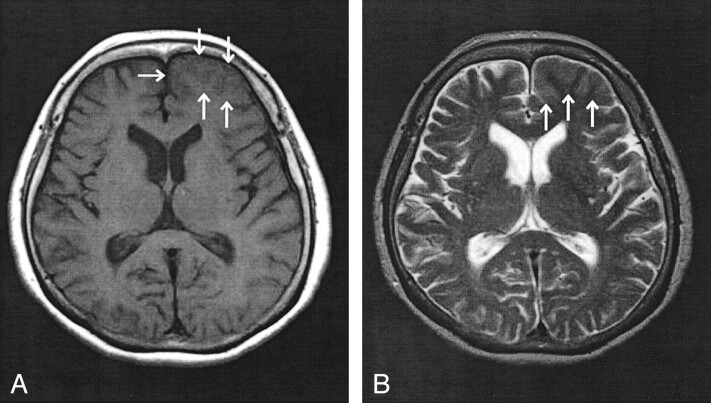
Fig 1.
Axial view MR images of the head, obtained at the time of first presentation.
A, T1-weighted MR image (400/15 [TR/TE]) shows effacement of the sulci and mild thickening of the cortex in the left frontal lobe (arrows).
B, T2-weighted MR image (3400/120) also shows effacement of the sulci and mild thickening of the cortex at the same location (arrows). Contrast material was not administered at this time.
Eight months later, the patient presented at our hospital with a right-sided clonic seizure involving the face and upper limb. Again, laboratory examination of the blood and CSF revealed no abnormality. Cytology of CSF was also negative. A physical examination disclosed no extracranial lesion. Repeat MR imaging of the head showed irregular thickening of the dura in the left frontal region. The lesion was isointense on T1-weighted images (Fig 2A) and enhanced with the administration of contrast material (Fig 2B). It showed low intensity on T2-weighted images, similar to that of the adjacent dura (Fig 2C). The lesion covered the surface of the left frontal lobe and involved the falx cerebri and the basal region. Sulci underlying the lesion were somewhat obscured. Angiography revealed the lesion to be mildly hypervascular and deriving its blood supply from the left anterior and posterior ethmoidal and the left frontopolar artery. A thallium scintigram showed a “hot spot” only at this same intracranial location. No extracranial involvement was noted on systemic gallium and thallium scintigrams.
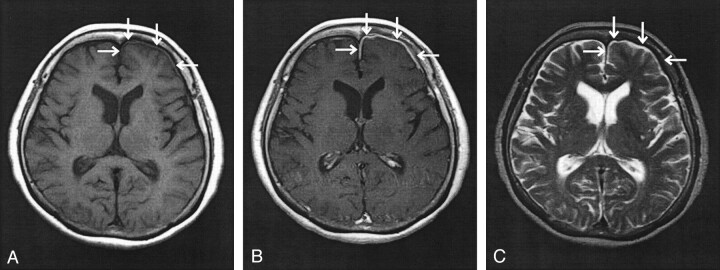
Fig 2.
Axial view MR images of the head, obtained at the time of second presentation.
A, T1-weighted MR image (400/15) shows thickening of the dura overlying the left frontal pull (arrows).
B, Contrast-enhanced T1-weighted MR image (400/15) shows enhancement of the dura overlying the left frontal lobe (arrows).
C, T2-weighted MR image (3400/150) shows reduction in signal intensity of the dura overlying the left frontal lobe (arrows), with no underlying abnormality within the parenchyma of the frontal lobe visualized.
An open biopsy was performed in August 2001 to rule out malignancy. The membranous lesion was milky white and rubbery. It was loosely adherent to both dura and pia, except at one focus where it showed firm adhesion to the cerebral cortex. Microscopically, the process to a far greater extent involved the subarachnoid space than the dural surface. It extended into the sulci, where the firm adhesion to the cerebral cortex was macroscopically present. It consisted of an attenuated infiltrate of lymphoplasmacytic cells and scattered, often multinucleate, histiocytic cells (Fig 3A). The lymphoplasmacytic cells were well differentiated without nuclear atypia. Although the histiocytes sometimes showed a loose indistinct sheetlike (granulomatous) arrangement, no epithelioid granuloma was present. The cytoplasm of some histiocytes was foamy, whereas the cytoplasms of others were pale and eosinophilic. The nucleus of histiocyte was not folded. The histiocytic cells occasionally showed emperipolesis (lymphocytophagocytosis) (Fig 3B). Although immunopositive for S-100 protein (Fig 3C), they were negative for CD1a.
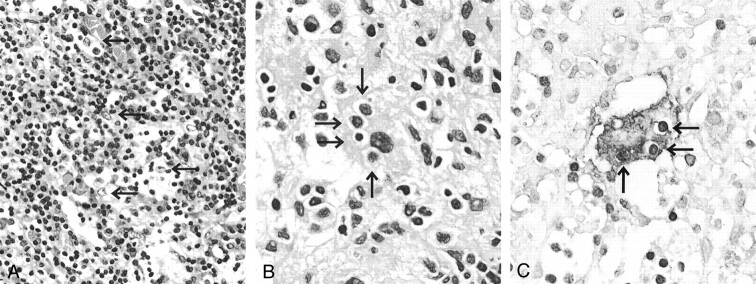
Fig 3.
Histopathology of the dural mass.
A, Lesion is composed of attenuated infiltrate of lymphoplasmacytic cells and histiocytic cells with pale granular cytoplasms (arrows).
B, Histiocytic cells occasionally show emperipolesis (lymphocytophagocytosis) (arrows).
C, Histiocytic cell with emperipolesis (arrows) is immunopositive for S-100 protein.
At the latest follow-up examination, performed in July 2002, the patient was well and receiving anti-epileptic medications. The residual lesion was radiographically stable in terms of size and extent.
Discussion
Among reported cases of extranodal Rosai-Dorfman disease, isolated intracranial lesions are extremely rare (3–8), are usually dura-based, and clinically mimic meningiomas (3–9). On T2-weighted MR images, meningiomas show low to high signal intensity, the variation being a reflection of histologic subtype (9). In contrast, Rosai-Dorfman disease shows a rather low signal intensity (3, 4, 10). On angiograms, meningiomas are commonly seen as hypervascular lesions (9). In contrast, the results are variable for Rosai-Dorfman disease (5, 7). Clinically, as do meningiomas, intracranial Rosai-Dorfman disease causes a variety of clinical symptoms depending on lesion location. Thus headache, epilepsy, cranial nerve deficits, and various other symptoms may be seen or become evident with disease progression. Frequent lesion locations include the cerebral convexities, the parasagittal, suprasellar, and cavernous sinuses, and petroclival regions (3–8). Histologically, Rosai-Dorfman disease is characterized by an attenuated infiltrate of lymphoplasmacytic cells and histiocytes of varying size. The large histiocytes often show emperipolesis (lymphocytophagocytosis). On immunohistochemical examination, these are positive for S-100 protein and negative for CD1a, a marker of Langerhans histiocytosis (2).
The immunophenotypic profiles and studies of monokine expression showed that the histiocytic cells of Rosai-Dorfman disease might derive from activate macrophages (11). It might be caused by an unusual response of the hematolymphoid system against an immunologic disorder (2); Epstein-Barr virus and human herpes virus six were detected by in situ hybridization in some cases (12). Although there have been some hypotheses regarding the pathogenesis of this rare lesion, it is still unclear.
In the present case, our clinical differential diagnosis included several diseases characterized by the capacity to undergo thin layer, extensive growth along, on, or in the dura (eg, en plaque and malignant meningioma, melanoma, metastatic carcinoma, infectious disease such as tuberculosis, and sarcoidosis). Once apparent, the dural mass showed low signal intensity on T2-weighted MR images and was found to derive its blood supply from the left frontopolar artery. In contrast, meningiomas are largely fed by a branch of the external carotid system (8). Although the feeding artery of intracranial Rosai-Dorfman disease is generally thought to correspond with its location, the finding of the feeding artery in our case was helpful to differentiate a meningioma. A malignant lesion was considered unlikely because the results of CSF cytology were normal. Furthermore, malignant lesions usually show accompanying edema in underlying brain parenchyma, as evidenced by high signal intensity on T2-weighted images (9). Infectious disease was also unlikely, considering that laboratory tests, including culture of both blood and CSF, were negative. It was very difficult to differentiate dura-based isolated meningioma-like tumefactive sarcoidosis clinically, because it may show low signal intensity on T2-weighted images and that may be enhanced with the administration of contrast material for T1-weighted MR imaging (13). This type of sarcoidosis may show no abnormalities on laboratory examinations of both blood and CSF. Furthermore, although Rosai-Dorfman disease may include a history of fever, malaise, night sweats, and weight loss for the patient with systemic involvement (2), isolated intracranial Rosai-Dorfman disease usually causes only neurologic symptoms (3–8). Obviously, a precise diagnosis could not be reached on clinical grounds alone.
The pathologic differential diagnosis of this particular case included lymphoma, plasma cell granuloma, and Langerhans cell histiocytosis. Lymphoma was unlikely, considering the lack of monotonous atypical lymphocytes. It may be difficult to distinguish Rosai-Dorfman disease from plasma cell granules in the absence of emperipolesis (lymphocytophagocytosis); previous reports of plasma cell granuloma encompassed both entities (14). Our preliminary, frozen section diagnosis was plasma cell granuloma. Langerhans cell histiocytosis was unlikely because Langerhans histiocytes with their familiar folded nuclei and eosinophil infiltrates were absent, as was immunoreactivity for CD1a, a reliable marker of Langerhans cell histiocytosis (2).
Although most of previously reported cases were of dura-based lesions mimicking meningiomas (3–8), they did not address subarachnoidal involvement. Our case showed a greater involvement in the subarachnoidal space than in the overlying dura. The abnormal imaging findings at the time of first presentation, which showed sulcal effacement and thickening of the cortex, might have corresponded with this histologic growth pattern. Moreover, these images could not show a distinct lesion at the dura. The lesion in our case might have originated from the subarachnoidal space, but this has not been proved.
Intracranial Rosai-Dorfman disease has been treated with surgery or other methods involving steroids, chemotherapeutic agents, and radiation (3–8, 10). Among these treatments, the surgical resection seems to be the most effective (3, 10). The prognosis of Rosai-Dorfman disease with involvement of the CNS was not poor. In a review of follow-up data of 43 patients, most patients (58%) were alive with disease (3). Only two patients (4.7%) had died (3). Moreover, no death was reported to have occurred as a result of isolated intracranial Rosai-Dorfman disease (4).
Conclusion
Although Rosai-Dorfman disease is a rare process, it should be included in the differential diagnoses for a dural mass mimicking meningioma (3–8). Furthermore, we should remember that Rosai-Dorfman disease may show an unusual imaging pattern, as it did in this case at the time of first presentation. Although neuroradiology might be of aid in the diagnosis of isolated intracranial Rosai-Dorfman disease, only histopathologic and immunocytochemical examinations permit a firm diagnosis.
Acknowledgments
We very much appreciate the assistance of Dr. Haruo Okazaki (Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN).
References
- 1.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol 1969;87:63–70 [PubMed] [Google Scholar]
- 2.Warnke RA, Weiss LM, Chan JK, Cleary ML, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). In: Rosai J, eds. Atlas of Tumor Pathology, 3rd Series: Tumors of the Lymph Nodes and Spleen. Washington: Armed Forces Institute of Pathology;1995. :349–360
- 3.Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-Dorfman disease isolated to the central nervous system: a report of 11 cases. Mod Pathol 2001;14:172–178 [DOI] [PubMed] [Google Scholar]
- 4.Kattner KA, Stroink AR, Roth TC, Lee JM. Rosai-Dorfman disease mimicking parasagittal meningioma: case presentation and review of literature. Surg Neurol 2000;53:452–457 [DOI] [PubMed] [Google Scholar]
- 5.Huang HY, Huang CC, Lui CC, Chen HJ, Chen WJ. Isolated intracranial Rosai-Dorfman disease: case report and literature review. Pathol Int 1998;48:396–402 [DOI] [PubMed] [Google Scholar]
- 6.Wu M, Anderson AE, Kahn LB. A report of intracranial Rosai-Dorfman disease with literature review. Ann Diagn Pathol 2001;5:96–102 [DOI] [PubMed] [Google Scholar]
- 7.Kitai R, Sato K, Kubota T, et al. Meningeal sinus histiocytosis mimicking lymphoplasmacyte-rich meningioma. J Neurosurg 1996;84:1051–1054 [DOI] [PubMed] [Google Scholar]
- 8.Deodhare SS, Ang LC, Bilbao JM. Isolated intracranial involvement in Rosai-Dorfman disease: a report of two cases and review of the literature. Arch Pathol Lab Med 1998;122:161–165 [PubMed] [Google Scholar]
- 9.Burger PC, Scheithauer BW. Tumor of meningothelial cells. In: Rosai J, eds. Atlas of Tumor Pathology, 3rd Series: Tumors of the Central Nervous System. Washington: Armed Forces Institute of Pathology,1995. :259–286
- 10.Udono H, Fukuyama K, Okamoto H, Tabuchi K. Rosai-Dorfman disease presenting multiple intracranial lesion with unique findings on magnetic resonance imaging. J Neurosurg 1999;91:335–339 [DOI] [PubMed] [Google Scholar]
- 11.Foss HD, Herbst H, Araujo I, et al. Monokine expression in Langhans’ cell histiocytosis and sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). J Pathol 1996;179:60–65 [DOI] [PubMed] [Google Scholar]
- 12.Levine PH, Jahan N, Murari P, Manak M, Jaffe ES. Detection of human herpesvirus 6 in tissues involved by sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). J Infect Dis 1992;166:291–295 [DOI] [PubMed] [Google Scholar]
- 13.Lexa FJ, Grossman RI. MR of sarcoidosis in the head and spine: spectrum of manifestations and radiographic response to steroid therapy. AJNR Am J Neuroradiol 1994;15:973–982 [PMC free article] [PubMed] [Google Scholar]
- 14.Burger PC, Scheithauer BW. Plasma cell granuloma. In: Rosai J, eds. Atlas of Tumor Pathology, 3rd Series: Tumors of the Central Nervous System. Washington: Armed Forces Institute of Pathology;1995. :407–408