Abstract
Summary: Currently available preparations of polymethylmethacrylate (PMMA) cement for percutaneous vertebroplasty have injectability times of 4–15 minutes. The potential for early polymerization requires procedures to be performed as fast as possible, sometimes with suboptimal results and waste of the cement. By cooling the PMMA mixture in an iced bath of sodium chloride solution, we can extend its injectability to over 2 hours and use one kit for the controlled treatment of multiple levels with successive needle placements. We have been using this technique since mid-1998, treating more than 600 vertebral bodies.
During percutaneous vertebroplasty, polymethylmethacrylate (PMMA) cement is injected into the vertebral body for the treatment of various painful spinal conditions, predominantly osteoporotic compression fractures. The cement reaction involves an exothermic reaction in which a liquid monomer cross-links with the powdered polymethylmethacrylate polymer to create a solid cement; the reaction releases 130 cal/g of monomer. Currently available preparations of PMMA cement for percutaneous vertebroplasty have injectability times in the range of 4 to 15 minutes (1). Those in orthopedic and dental circles have long known that the setting rate of PMMA cement can be changed by increasing the monomer-to-powder ratio, by decreasing the temperature, or by chilling the liquid monomer (2).
Because it confers structural stabilization, vertebroplasty has become an accepted treatment for the unremitting pain associated with vertebral compression fractures (3). Controlled cement injection under fluoroscopic guidance minimizes the possibility of substantial, errant deposition into the spinal canal or neural foramen and the limits the potential for distal embolization to the lung. However, procedures must be performed as quickly as possible to prevent premature hardening of the cement within the needle or delivery system, which might lead to suboptimal results with underfilling of the vertebrae. We have found that we can retard the polymerization process by cooling the mixture to allow us to comfortably inject cement by using a 1-mL syringe in as many as three levels with only one mixing. We empirically compared injectability of materials stored at two different temperature settings.
Technique
In this experiment, we used our standard PMMA mixture consisting of one package of type 1–Slow Set Codman Cranioplastic (Depuy CMW, Blackpool, UK). We mixed approximately 27–28 of the 30 g of supplied powder (about 1 tsp left out), 17 mL of the supplied liquid monomer, 6 g of sterile barium (Bryan Corporation, Woburn, MA) and 1.2 g of tobramycin powder (Eli Lilly and Company, Indianapolis, IN). The mixture was prepared under mild vacuum conditions in a Stryker Mixevac II mixing chamber (Ultramix; DePuy Inc., Warsaw, IN). The PMMA preparation was drawn up in a liquid form into four 12-mL standard luer-lock syringes and capped. Two of the syringes were immediately submerged in an iced bath of sterile sodium chloride solution (Fig 1). These were frequently rotated to keep the mixtures as evenly cooled as possible. The iced sodium chloride solution was replenished as the ice melted. The other two syringes were kept at ambient room temperature. The ice bath and room temperatures were recorded as 33°F and 68°F, respectively, by using a simple temperature probe. The injectability was tested at 5, 15, 25, 30, 60, 90, 120, and 150 minutes. Photographs (Fig 2A and B) were taken at these intervals to graphically demonstrate the viscosity and ease of injection. At room temperature, the cement began hardening at about 25 minutes, rapidly becoming uninjectable at 30 minutes. In contrast, cement immersed in the iced sodium chloride solution was injectable for as long as 2 hours 15 minutes and remained injectable with difficulty at 2 hours 30 minutes.
Fig 1.
Iced sodium chloride bath. PMMA cement is mixed, loaded, and capped in 12-mL luer-lock syringes, which are kept in an iced sodium chloride bath in a small 35-oz solution bowl. The syringes were rotated to keep them evenly submersed and cooled.
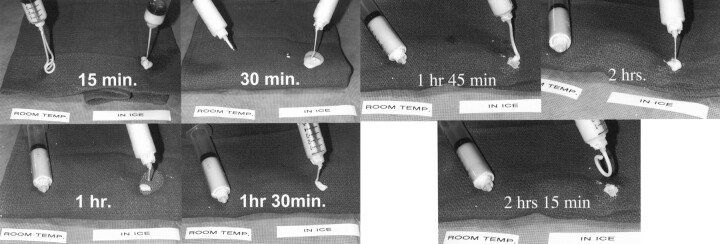
Fig 2.
Photographic demonstration of the injectability of the cement at room temperature (left syringe in each image) and the cement immersed in the iced sodium chloride solution (right syringe in each image). At 30 minutes, the syringe at room temperature has hardened and is impossible to inject compared with freely flowing cooled mixture. The mixture begins to become semisolid at 2 hours 15 minutes.
Discussion
Early in our practice of percutaneous vertebroplasty, we noted problems with early polymerization and premature setting of the PMMA cement; on more than one occasion, these caused suboptimal filling of a compressed vertebral body. In addition, we have often used more than one kit for a single procedure. Noting that the polymerization reaction was an exothermic one, we hypothesized that by chilling the mixture we could substantially retard the setting process. The Arrhenius (4) rate equation predicts an approximate doubling of the reaction rate for each 10°C increase in temperature; we experimented with placing the mixture in an ice bath. We noted a substantial retardation of the polymerization process, which allowed the PMMA mixture to remain injectable for as long as 2 hours 30 minutes. Our experiment yielded an eightfold increase in polymerization time, compared with that conservatively suggested by the manufacturer.
Since mid-1998, we have used this process and have been able to treat more than 600 compression fractures with no major complications. We use 1-mL syringes and inject small increments of only 0.1–0.2 mL Using this technique, we have been able to inject as many as three levels in one sitting. The associated predictability has improved results and outcomes in our patients. We have had no clinically important extension of the cement outside the vertebral body.
Although the effects of the operating-room temperature and chilling of the liquid monomer have been discussed as being relevant to orthopedic molding procedures, our technique and application to percutaneous vertebroplasty has not been reported, to our knowledge. Studies have shown that there the actual strength of the final products formed at these temperatures is not notably different (5). Jasper et al (6) have shown that increasing the monomer-to-powder ratio decreases the viscosity and also the mechanical strength by as much as 24%. However, whether these effects are clinically important is unknown. The absolute time of injectability may vary considerably, depending on the different PMMA preparations and the needle bore chosen.
Our clinical experience proved the obvious benefits of less waste and better results associated with a delayed polymerization time; in this case, the time was increased as much as eightfold. Icing the mixture is an important contribution to improving the technique and safety of vertebroplasty. As newer cements are developed, we believe that this general principal should be kept in mind.
References
- 1.Cranioplastic TM, Type 1-Slow-set, LCN 183032-001/J, Codman & Schurtleff, Inc., 1999
- 2.Pearson GP, Jones DF, Wright V. Letter: Effect of operating theatre temperatures on the setting times of acrylic cements for use in orthopaedic surgery. Lancet 1975;2:184. [DOI] [PubMed] [Google Scholar]
- 3.Mathis JM, Barr JD, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–381 [PMC free article] [PubMed] [Google Scholar]
- 4.Eggers DF, Gregory NW, Halsey GD, Rabinovitch BS. Physical Chemistry. New York: John Lily and Son; 1964:435
- 5.Parks ML, Walsh HA, Salvati EA, Li S. Effect of increasing temperature on the properties of four bone cements. Clin Orthop 1998;355:238–248 [DOI] [PubMed] [Google Scholar]
- 6.Jasper LE, Deramond H, Mathis JM, Belkoff SM. The effect of monomer-to-powder ratio on the material properties of cranioplastic. Bone 1999;25(suppl 2):27S–29S [DOI] [PubMed] [Google Scholar]