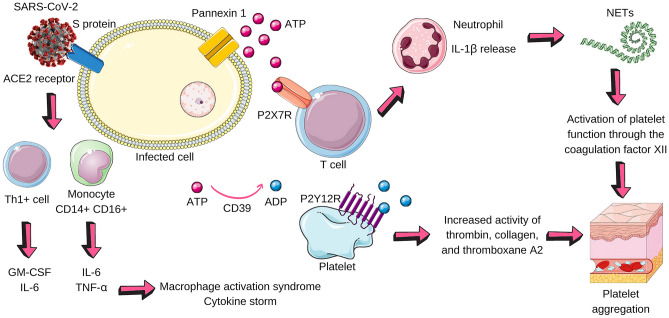
Fig. 2.
Possible purinergic pathways in COVID-19. SARS-CoV-2 infects the cell (pneumocyte, for example) from the interaction of its surface protein with the ACE2 receptor present on the cell surface. This link activates Th1 + cells, which release GM-CSF and IL-6, and monocytes, which secrete IL-6 and TNF-alpha. The microenvironment becomes pro-inflammatory and two important phenomena happen: macrophage activation syndrome (MAS) and the cytokine storm. The infected cell, in a state of stress, releases ATP through pannexin 1. ATP binds to the P2X7 receptor that promotes the release of IL-1β by neutrophils. CD39 dephosphorylates ATP in ADP, which acts on the P2Y12 receptor on platelets and has the following effects: to increase the activity of thrombin, collagen, and thromboxane A2. In addition to these factors that contribute to platelet aggregation, neutrophils release NETs that activate platelet factor XII. The result is platelet aggregation, excessive release of cytokines, and an inflammatory microenvironment. SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2, ACE2 angiotensin-converting enzyme 2, GM-CSF macrophage colony-stimulating factor, IL-6 interleukin-6, TNF-alpha tumor necrosis factor alpha, ATP adenosine triphosphate, IL-1B Interleukin 1 beta, CD39 Cluster of Differentiation 39, ADP adenosine diphosphate, NETs neutrophil extracellular traps