Abstract
BACKGROUND AND PURPOSE: Intracranial aneurysms are common, with an overall frequency ranging from 0.8% to 10%. Because prognosis after subarachnoid hemorrhage is still very poor, treatment of unruptured aneurysms, either neurosurgically or endovascularly, has been advocated. However, risk of rupture and subsequent subarachnoid hemorrhage needs to be considered against the risks of elective treatment. We analyzed the technical feasibility, safety, and efficacy of endovascular treatment of a consecutive series of unruptured cerebral aneurysms.
METHODS: From July 1997 through December 2000, a total of 76 patients with 82 unruptured cerebral aneurysms were treated at our institution. Endovascular treatment was administered to 39 consecutive patients with a total of 42 unruptured cerebral aneurysms. Thirty-six aneurysms were treated with an endosaccular technique; in six patients, the parent artery was occluded to eliminate aneurysmal perfusion. Aneurysms were located either in the anterior (n = 31) or posterior (n = 11) circulation. Eight patients had experienced previous subarachnoid hemorrhage from other aneurysms and were treated electively after complete rehabilitation. Ten patients had neurologic symptoms; in 21 patients, the aneurysm was an incidental finding. Eighteen aneurysms were small (0–5 mm), 11 were medium (6–10 mm), nine were large (11–25 mm), and four were giant (> 25 mm). Occlusion rate was categorized as complete (100%), subtotal (95–99%), and incomplete (< 95%) obliteration.
RESULTS: Endovascular treatment was technically feasible for 38 of 42 aneurysms. Complete (100%) or nearly complete (95–99%) occlusion was achieved in 34 of 38 aneurysms. In four aneurysms of the internal carotid artery, only incomplete (< 95%) occlusion was achieved. All patients except one with mild neurologic deficits according to the Glasgow Outcome Scale and one with mild memory dysfunction but no focal neurologic deficit achieved good recovery, resulting in a morbidity rate of 4.8% and a mortality rate of 0%.
CONCLUSION: Endovascular embolization of unruptured cerebral aneurysms is an effective therapeutic alternative to neurosurgical clipping and is associated with low morbidity and mortality rates. For the management of unruptured aneurysms, endovascular treatment should be considered.
Intracranial aneurysms are common. Autopsy studies have shown that the overall frequency in the general population ranges from 0.8% to 10% (1–4). Management of intracranial aneurysms has improved significantly during recent years because of major technical advances. However, prognosis after subarachnoid hemorrhage is still very poor. The optimal management of unruptured intracranial aneurysms remains controversial. This is because of a lack of understanding of the natural history of intracranial aneurysms and the published results regarding procedural complications associated with neurosurgical and endovascular treatments (5–8). Management decisions require an accurate assessment of the risks of treatment options compared with the natural history of the pathologic abnormality. To analyze the technical feasibility, safety, and efficacy of endovascular treatment of unruptured cerebral aneurysms, we reviewed our consecutive series.
Methods
From July 1997 through December 2000, a total of 76 patients with 82 unruptured cerebral aneurysms were treated at our institution. Endovascular treatment was administered to 39 consecutive patients (16 male and 23 female patients) with a total of 42 unruptured cerebral aneurysms. There were no standard selection criteria, whether the patient was treated with endovascular techniques or with neurosurgical clipping. Every treatment was jointly discussed in an interdisciplinary manner among neuroradiologists and neurosurgeons. We discussed both treatment modalities for every aneurysm with the patient. In patients with aneurysms in the posterior circulation and in patients with infraclinoidal or giant aneurysms, we favored endovascular techniques because of the more complicated or impossible surgical approach. In aneurysms for which either endovascular or neurosurgical treatment seemed appropriate, the patients chose to be treated via an endovascular route.
The mean patient age was 47.8 years (range, 9–76 years), including three children who were 9, 11, and 12 years old. Aneurysms were located in the anterior circulation (n = 31), with six arising from the anterior communicating artery, one from the pericallosal artery, four from the middle cerebral artery, and 20 from the internal carotid artery. In the posterior circulation (n = 11), seven occurred at the basilar artery tip, one at the basilar trunk, two at the posterior inferior cerebellar artery, and one at the vertebral artery. For details, see Table 1.
Patient, aneurysm, and treatment data
| Patient No. | Aneurysm No. | Patient Age (y) | Sex | Aneurysm Location | Aneurysm Size | 95–100% (+)<95% (−) |
|---|---|---|---|---|---|---|
| 1 | 1 | 48 | F | ICA | 6 | + |
| 2 | ICA | 3 | Failure | |||
| 2 | 3 | 34 | F | ICA | 4 | + |
| 3 | 4 | 47 | F | AcoA | 2 | + |
| 4 | 5 | 46 | F | ICA | 7 | − |
| 5 | 6 | 58 | F | ICA | 40 | + |
| 6 | 7 | 48 | M | ICA | 10 | − |
| 7 | 8 | 61 | M | AcoA | 7 | + |
| 8 | 9 | 62 | F | AcoA | 3 | + |
| 9 | 10 | 46 | M | MCA | 3 | + |
| 10 | 11 | 37 | F | ICA | 13 | − |
| 11 | 12 | 42 | M | BA tip | 3 | + |
| 13 | BA trunk | 15 | + | |||
| 12 | 14 | 60 | M | MCA | 7 | + |
| 13 | 15 | 45 | F | BA tip | 6 | + |
| 14 | 16 | 41 | F | ICA | 4 | + |
| 15 | 17 | 67 | F | ICA | 30 | + |
| 16 | 18 | 41 | M | AcoA | 10 | + |
| 17 | 19 | 62 | M | AcoA | 7 | + |
| 18 | 20 | 39 | F | ICA | 7 | + |
| 19 | 21 | 76 | F | ICA | 25 | + |
| 20 | 22 | 58 | M | ACA | 4 | + |
| 21 | 23 | 59 | M | BA tip | 11 | + |
| 22 | 24 | 31 | M | MCA | 5 | + |
| 23 | 25 | 51 | F | ICA | 6 | + |
| 24 | 26 | 74 | F | BA tip | 11 | + |
| 25 | 27 | 52 | M | ICA | 20 | + |
| 26 | 28 | 74 | F | ICA | 3 | + |
| 27 | 29 | 39 | F | ICA | 10 | − |
| 28 | 30 | 40 | F | ICA | 3 | Failure |
| 29 | 31 | 33 | F | ICA | 3 | + |
| 30 | 32 | 11 | M | ICA | 25 | + |
| 31 | 33 | 47 | M | AcoA | 4 | + |
| 32 | 34 | 48 | F | BA tip | 12 | + |
| 33 | 35 | 58 | M | BA tip | 4 | + |
| 34 | 36 | 62 | F | MCA | 12 | + |
| 35 | 37 | 40 | F | ICA | 5 | Failure |
| 36 | 38 | 71 | F | PICA | 4 | Failure |
| 37 | 39 | 9 | M | VA | 40 | + |
| 40 | PICA | 4 | + | |||
| 38 | 41 | 12 | M | ICA | 35 | + |
| 39 | 42 | 34 | F | BA tip | 3 | + |
Note.—ICA indicates internal carotid artery; AcoA, anterior communicating artery; MCA, middle cerebral artery; BA, basilar artery; PICA, posterior inferior cerebellar artery; VA, vertebral artery; 95–100%, subtotal to complete obliteration; <95%, incomplete obliteration.
Eight patients had experienced previous subarachnoid hemorrhage from other aneurysms and were treated electively after complete rehabilitation. Seven patients had neurologic symptoms, including cranial nerve palsy, visual disturbances, and brain stem compression due to the mass effect of the aneurysm itself. In 24 patients examined because of previous infarction, headache, or dizziness and benign intracranial tumors (n = 3), the aneurysm was an incidental finding.
Eighteen aneurysms were small (0–5 mm), 11 were medium (6–10 mm), nine were large (11–25 mm), and four were giant (>25 mm). Treatment modality, either neurosurgical or endovascular, was determined jointly in an interdisciplinary approach by neuroradiologists and neurosurgeons. Decision criteria included location and size of the aneurysm, patient age, other medical conditions, and patient choice.
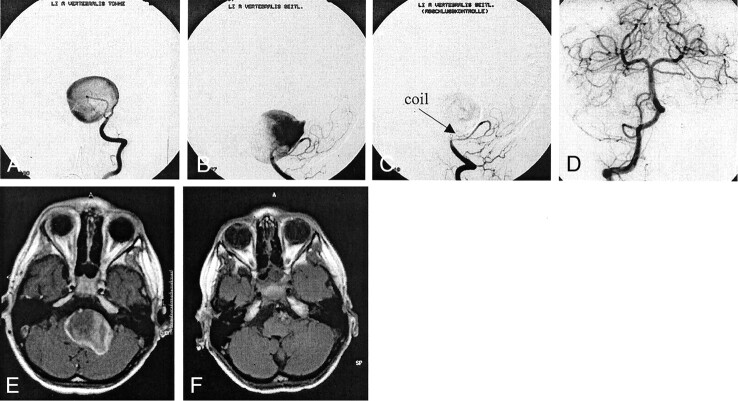
It was decided that 36 of the aneurysms should be treated by filling the aneurysmal sack with platinum coils. Occlusion of the parent artery was deliberately performed in six aneurysms to eliminate aneurysmal perfusion. To occlude the vessel, we used detachable balloons in two patients and platinum coils in four. Three of these patients had giant and two had large broad based aneurysms of the internal carotid artery; one had a giant vertebral artery aneurysm (FIG 1).
Fig 1.
Case 2. Images of a 9-year-old male patient with a giant left vertebral aneurysm.
A, Towne projection.
B, Lateral projection.
C, After placing a coil at the neck of the aneurysm, complete elimination was achieved.
D, No retrograde filling was observed during injection into the right vertebral artery.
E and F, Cross-sectional images show complete regression of brain stem compression.
All patients were treated while under general anesthesia and received IV administered heparin during and until 72 hr after the procedure. After 3 days, aspirin (100 mg/day administered orally) was started and continued for 3 months.
For technical feasibility, we documented the ability to maneuver the microcatheter into the aneurysmal sack, to introduce platinum coils, and to occlude the vessel in cases with deliberate parent vessel occlusion. Evaluation of efficacy, as determined by the extent of aneurysm occlusion, was conducted immediately after intervention and at follow-up angiography. Occlusion rate was categorized as complete (100%), subtotal (95–99%), and incomplete (<95%) obliteration of the aneurysm. In terms of safety, we documented the amount of procedural complications and assessed clinical outcome according the Glasgow Outcome Scale. Every patient was scheduled to undergo follow-up angiography (digital subtraction and MR angiography) and clinical evaluation 6 months after treatment.
Results
Endovascular treatment was technically feasible in 38 of 42 aneurysms. In four patients, endovascular treatment failed. This was because of the unfavorable dome-to-neck ratio in three aneurysms. In one of the three aneurysms, located at the distal internal carotid artery (Table 1, patient 1), neurosurgical clipping was successfully performed. The other two aneurysms were not considered for clipping because of the infraclinoidal location of the neck. Selective catheterization of a small aneurysm at the origin of the posterior inferior cerebellar artery in a 71-year-old woman could not be achieved because of severe elongation of the subclavian and proximal vertebral artery. In this case, direct puncture of the vertebral artery was discussed but the patient refused further intervention.
Complete (100%) or nearly complete (95–99%) occlusion was achieved in 34 aneurysms. In four (three medium and one large) aneurysms of the internal carotid artery, only incomplete (<95%) occlusion was achieved. In two of these aneurysms, the residual part of the aneurysm was extradural and will be followed.
Procedural complications included one case of thromboembolic vessel occlusion due to narrowing of the parent artery induced by slight coil protrusion, resulting in a partial infarction of the anterior cerebral artery territory (Fig 2). An initial hemiparesis led to a mild spastic paresis of the leg, leaving the patient totally independent and not restricting him from playing golf. No other complications were observed during endovascular intervention. In particular, no aneurysmal rupture occurred.
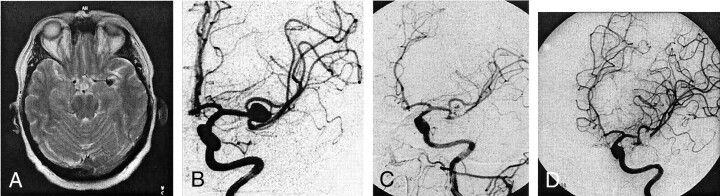
Fig 2.
Case 3. Images of a 61-year-old male patient with a medium anterior communicating artery aneurysm.
A, Before intervention.
B, After intervention, complete obliteration is achieved.
C, Two hours after intervention, thrombus is revealed in the proximal A2 segment. Intra-arterial lysis was performed with 20 mg of recombinant tissue type plasminogen activator.
D, Control angiogram shows partial recanalization, with marked improvement of anterior perfusion.
E and F, MR images reveal partial infarction in the anterior territory.
After endovascular occlusion of the internal carotid artery proximal to a large aneurysm, another patient developed mild intermittent hemiparesis, probably due to stump emboli. That aneurysm could not be trapped. The symptoms resolved completely before the patient was discharged.
After complete embolization of a medium aneurysm of the anterior communicating artery, the patient had memory dysfunction but no focal neurologic deficit. The symptoms in this patient improved markedly during a period of 6 months, at which time he returned to undergo routine angiography, which showed complete obliteration of the aneurysm.
Of seven patients with neurologic deficits before endovascular treatment, symptoms resolved completely in four, improved in two, and did not change in one young patient with long-standing visual disturbance due to a giant aneurysm of the internal carotid artery compressing the optic nerve (Table 1, patient 38). In six of these seven patients, parent artery occlusion was achieved to eliminate aneurysmal flow; in one patient with a IIIrd nerve palsy, the aneurysmal sack was embolized with coils and symptoms resolved completely. Five patients with infarction before intervention had unchanged neurologic conditions after the procedure.
In total, according to the Glasgow Outcome Scale, all achieved good recovery except one patient with mild deficits and one patient with mild memory dysfunction, resulting in an overall morbidity rate of 4.8%. No procedure-related death occurred, and no intracranial bleeding was observed during follow-up.
During the follow-up period (≥6 months; maximum, 33 months) 23 of 38 aneurysms remained completely eliminated, including those in which the parent artery was occluded. Four aneurysms that were initially incompletely occluded revealed aneurysmal flow after 6 months. In one female patient with an aneurysm at the middle cerebral artery bifurcation, we observed some remaining opacification at the center of the aneurysm at the end of endovascular intervention. The follow-up angiogram obtained 6 months later revealed complete occlusion (Fig 3). Eleven patients did not have control angiograms available, seven because they refused and four because they had been treated within the last 6 months.
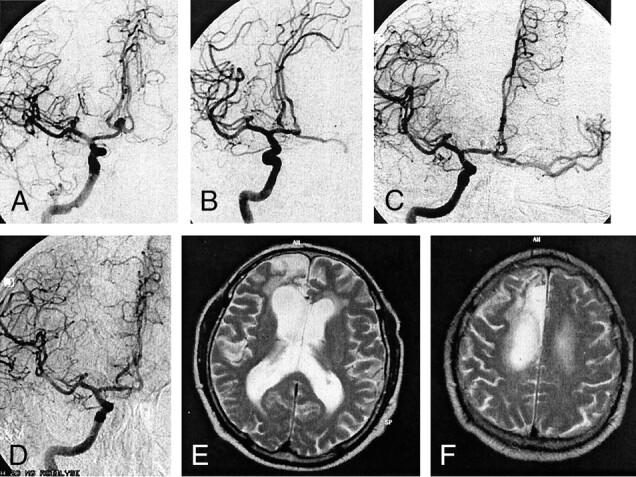
Fig 3.
Case 1. Images of a 62-year-old female patient with headache.
A, T2-weighted MR image shows possible left middle cerebral artery aneurysm.
B, Digital subtraction angiogram confirms left middle cerebral artery aneurysm.
C, Immediately after endovascular embolization, aneurysmal flow in the center is still observed.
D, Follow-up angiogram obtained 6 months later shows complete obliteration of the aneurysm.
Illustrative Cases
Case 1 was that of a 9-year-old male patient who presented with nausea and vomiting. MR imaging revealed a giant aneurysm in the posterior fossa, with marked compression of the brain stem. Conventional angiography showed the aneurysm arising from the vertebral artery distal to the origin of the posterior inferior cerebellar artery. One vortex coil (2/4 mm) was positioned at the narrowed neck of the aneurysm, and inflow from the left vertebral artery was eliminated. Control angiography with injection into the right vertebral artery did not show any retrograde filling of the aneurysm. Follow-up cross-sectional MR imaging revealed complete thrombosis of the aneurysm, which subsequently completely disappeared. The brain stem symptoms resolved completely.
Case 2 was that of a 62-year-old female patient who experienced sudden loss of consciousness but who had no history of subarachnoid hemorrhage and no other medical impairment. MR imaging revealed a large aneurysm of the left middle cerebral artery, with a diameter of 12 mm. After filling the aneurysmal sack with four platinum coils (Guglielmi detachable coil 10: 8/30, 7/30, 6/20, and 6/20 mm), there was still a small inflow into the center of the aneurysm. Control angiography performed 6 months after intervention revealed subsequent thrombosis of the aneurysm with complete obliteration. No deficit remained.
Case 3 was that of a 61-year-old male patient who experienced one episode of dizziness. He underwent MR imaging, which revealed an anterior communicating artery aneurysm. He had no history of subarachnoid hemorrhage. The medium aneurysm, with an estimated diameter of 7 mm, was occluded with five platinum coils (Guglielmi detachable coil 10: 6/20, 5/15, 4/6, 4/4, and 3/6 mm). Two hours after intervention, the patient experienced left hemiparesis. Conventional CT performed at that time did not reveal any infarction, whereas conventional digital subtraction angiography revealed a thrombus in the right A2 segment. After intra-arterial infusion of 20 mg of recombinant tissue type plasminogen activator, partial recanalization and marked improvement of the right anterior perfusion were observed. Follow-up MR imaging revealed partial infarction in the right anterior territory. After 6 months, the hemiparesis resolved almost completely; residual mild spastic paresis remained in the leg, but the patient was totally independent and was not restricted from playing golf.
Discussion
Despite recent advances in the management of intracranial aneurysms, subarachnoid hemorrhage from a ruptured intracranial aneurysm is often a devastating event and the overall outcome, with high morbidity and mortality rates, remains unsatisfactory. However, the natural history of unruptured intracranial aneurysms is still unclear but is obviously influenced by many factors, such as previous subarachnoid hemorrhage, patient age, coexisting medical conditions, and aneurysm characteristics such as size, location, and morphology. Additionally, the professional skills and experience of the neurovascular team have a major impact on treatment decisions (5, 9–16). These different factors have contributed to a considerable variability in the reported risks for aneurysmal subarachnoid hemorrhage and treatment of unruptured intracranial aneurysms. No prospective randomized trials of treatment interventions versus conservative management have been conducted to date.
Our results showed very low morbidity and mortality rates associated with the endovascular treatment of unruptured intracranial aneurysms. Technical feasibility in >90% of our cases and a high occlusion rate justify comparison with results of neurosurgical treatment of unruptured aneurysms. Evaluation of occlusion rate was conducted immediately after intervention and reevaluation of more than half the cases was conducted ≥6 six months later. We note that the data of our series do not reflect long-term results.
No procedure-related death occurred in our series, which had a procedural morbidity rate comparable with that of the 733-patient meta-analysis conducted by King et al (17) in 1994 (4.8% versus 4%, respectively). Our study had lower morbidity and mortality rates than those of the meta-analysis conducted by Raaymakers et al (7) in 1998. They reported a morbidity rate of 10.9% and a mortality rate of 2.6% for neurosurgically treated unruptured aneurysms in 2460 patients. Murayama et al (18) reported a morbidity rate of 4.3% for a total of 109 patients, with no morbidity occurring in the last 65 patients after endovascular treatment of unruptured aneurysms. In the retrospective study presented by Johnston et al (19), significantly fewer complications occurred after coiling than after surgical clipping. However, these numbers should be considered with caution because they are averaged and do not reflect the individual skill of the neurosurgeon. The same is true for the endovascular approach. Therefore, one has to carefully discuss treatment options in an interdisciplinary manner for every patient at every institution.
Whether to treat unruptured aneurysms, especially small unruptured aneurysms, remains controversial. Since the publication of the study conducted by The International Study of Unruptured Intracranial Aneurysms Investigators (15), there has been much discussion concerning the treatment of unruptured aneurysms <10 mm.
Health care is currently undergoing major reorganization to meet growing economic pressure; consequently, the aspect of preventative therapy becomes more and more important. Therefore, aneurysm treatment must be considered in several respects. What is the risk of aneurysm rupture? What are the costs associated with treating subarachnoid hemorrhage? What are the costs associated with treating unruptured aneurysms, either neurosurgically or via an endovascular approach, to avoid subarachnoid hemorrhage and its possible fatal complications? Costs associated with treating aneurysmal hemorrhage need to be considered in conjunction with the risk of rupture of an incidentally detected aneurysms.
Recent articles (14, 18, 20–23) comparing surgical and endovascular treatment of unruptured aneurysms have reported that the costs associated with treating an unruptured aneurysm are significantly lower than those associated with treating subarachnoid hemorrhage, based on length of hospital stay and morbidity rate. By comparing the results of surgical clipping and coil embolization at 60 university hospitals, Johnston et al (20) reported significantly higher costs ($43,000 versus $30,000) and significantly greater lengths of hospital stay (9.6 days versus 4.6 days) for the surgical cases.
It is necessary to provide the patient with all treatment options. Considering cost-effectiveness and that endovascular treatment has lower morbidity and mortality rates than does neurosurgery, unruptured cerebral aneurysms in any location should be considered first for endovascular treatment.
Conclusion
In our experience, the endovascular treatment of unruptured cerebral aneurysms is safe and effective. It represents a valid alternative to surgical clipping.
References
- 1.Chason J, Hindman W. Berry aneurysms of the circle of Willis: results of a planned autopsy study. Neurology 1958;8:41–44 [DOI] [PubMed] [Google Scholar]
- 2.Housepian E, Pool J. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol 1958;17:409–423 [DOI] [PubMed] [Google Scholar]
- 3.Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol 1990;34:361–365 [DOI] [PubMed] [Google Scholar]
- 4.McCormick W, Acosta-Rua G. The size of intracranial saccular aneurysms: an autopsy study. J Neurosurg 1970;33:422–427 [DOI] [PubMed] [Google Scholar]
- 5.Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg 1993;79:174–182 [DOI] [PubMed] [Google Scholar]
- 6.Orz Y, Hongo K, Tanaka Y, et al. Risks of surgery for patients with unruptured intracranial aneurysms. Surg Neurol 2000;53:21–29 [DOI] [PubMed] [Google Scholar]
- 7.Raaymakers T, Rinkel G, Limburg M, Algra A. Mortality and morbidity of surgery for unruptured intracranial anreurysms. Stroke 1998;29:1531–1538 [DOI] [PubMed] [Google Scholar]
- 8.Tomasello F, D’Avella D, Salpietro F, Longo M. Asymptomatic aneurysms: literature meta-analysis and indications for treatment. J Neurosurg Sci 1998;42:47–51 [PubMed] [Google Scholar]
- 9.Bederson J, Awad I, Wiebers D, et al. Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke 2000;31:2742–2750 [DOI] [PubMed] [Google Scholar]
- 10.Carrizo A. Epidemiological features and diagnostic evaluation of intracranial aneurysms. Crit Rev Neurosurg 1999;9:79–86 [DOI] [PubMed] [Google Scholar]
- 11.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms. Stroke 2001;32:485–491 [DOI] [PubMed] [Google Scholar]
- 12.Juvela S. Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke 2001;32:815. [DOI] [PubMed] [Google Scholar]
- 13.Rinkel F, Djibuti M, van Gijn J. Prevalence and risk of ruptured of intracranial aneurysms: a systematic review. Stroke 1998;29:251–256 [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw J, White P. The detection and management of unruptured intracranial aneurysms. Brain 2000;123:205–221 [DOI] [PubMed] [Google Scholar]
- 15.The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med 1998;339:1725–1733 [DOI] [PubMed] [Google Scholar]
- 16.Yasui N, Suzuki A, Nishimura H, Suzuki K, Abe T. Long-term follow-up study of unruptured intracranial aneurysms. Neurosurgery 1997;40:1155–1160 [DOI] [PubMed] [Google Scholar]
- 17.King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg 1994;81:837–842 [DOI] [PubMed] [Google Scholar]
- 18.Murayama Y, ViñAtnuela F, Duckwiler G, Gobin P, Guglielmi G. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg 1999;90:207–214 [DOI] [PubMed] [Google Scholar]
- 19.Johnston SC, Wilson C, Halbach V, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol 2000;48:11–19 [DOI] [PubMed] [Google Scholar]
- 20.Johnston SC, Dudley RA, Gress DR, Ono L. Surgical and endovascular treatment of unruptured cerebral aneurysms at university hospitals. Neurology 1999;52:1799–1805 [DOI] [PubMed] [Google Scholar]
- 21.Johnston SC, Gress D, Kahn J. Which unruptured cerebral aneurysms should be treated? a cost-utility analysis. Neurology 1999;52:1806–1815 [DOI] [PubMed] [Google Scholar]
- 22.Johnston SC. Effect on endovascular services and hospital volume on cerebral aneurysm treatment outcomes. Stroke 2000;31:111–117 [DOI] [PubMed] [Google Scholar]
- 23.Wiebers DO, Torner JC, Meissner I. Impact of unruptured intracranial aneurysms on public health in the United States. Stroke 1992;23:1416–1419 [DOI] [PubMed] [Google Scholar]