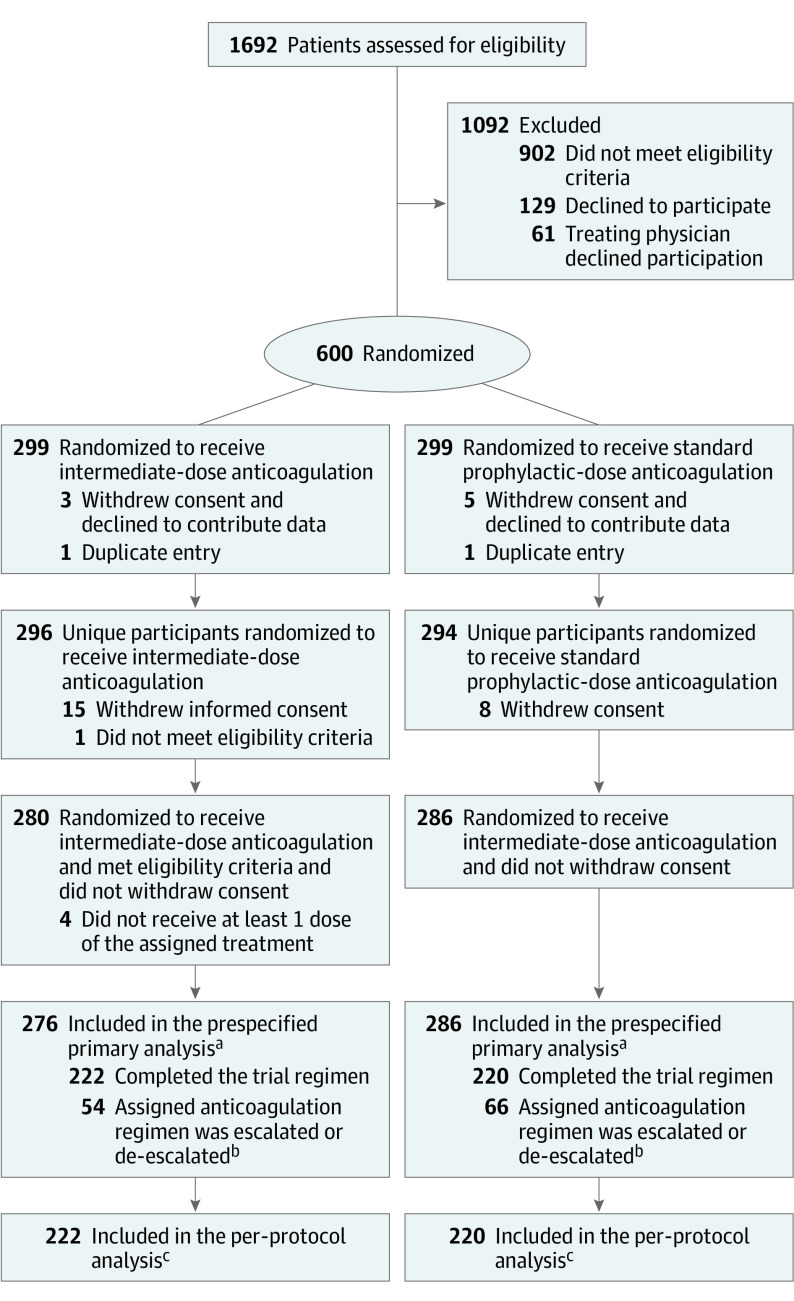
Figure 1. Flow of Participants in a Study of the Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation Among Patients With COVID-19 Admitted to the Intensive Care Unit .
aSome patients had more than 1 reason for exclusion from the primary analysis cohort.
bReasons for discontinuation of the trial regimen are summarized in eTable 4 in Supplement 3.
cPatients who were randomized and were not excluded due to violation of the eligibility criteria or withdrawal of informed consent and continued their assigned anticoagulation regimen until 30-day follow-up or the occurrence of the prespecified efficacy outcome. See eTable 13 in Supplement 3 for the per-protocol safety cohort.