Abstract
BACKGROUND AND PURPOSE: Time-of-flight MR angiography (TOF MRA), with its advantage of high spatial resolution, is widely used for visualization of intracranial arteries. Fat signal intensity from bone marrow can interfere with vessel signal intensity, especially in maximum intensity projection reconstructions. Use of a technique such as fat saturation or water excitation can reduce this signal intensity.
METHODS: Ten volunteers were included in this study. TOF MRA was performed by using spoiled gradient echo sequences on a 1.5-T MR unit either with or without water excitation. Water excitation was performed by using a binomial excitation pulse pair, with a null in the excitation profile at the fat frequency to obtain fat suppression. Two blinded neuroradiologists then judged the images. Additional studies by using a phantom with a flow of about 2 mL/s were performed under the same conditions. Image quality obtained with and that without water excitation was graded as similar by both neuroradiologists.
RESULTS: As the main finding, the sequences by using water excitation revealed an important pitfall: apparent carotid artery stenosis was detected in 4/10 and occlusions in 1/10 cases. Use of a flow phantom could reveal the same pitfall. Intracranial vessel disease was excluded in all volunteers by using Doppler sonography.
CONCLUSION: The type of water excitation tested here can induce significant artifacts in cerebral TOF MRA. These artifacts can be misinterpreted as carotid artery stenosis or even occlusion, whereas the benefit brought by water excitation with respect to fat suppression is less significant.
Time-of-flight MR angiography (TOF MRA) is widely used for visualization of intracranial arteries. The main advantage is its high spatial resolution compared with contrast-enhanced MRA by using spoiled gradient echo (FLASH) sequences (1, 2).
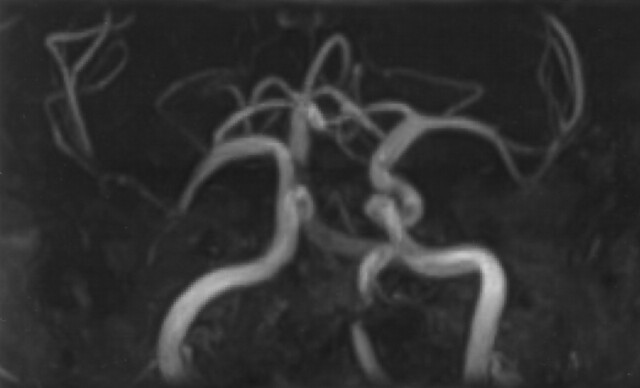
With TOF MRA, the high signal intensity of bone marrow fat can interfere with vessel depiction, especially in maximum intensity projection (MIP) reconstructions (3) (Fig 1). A chemically selective fat presaturation pulse is useful for improving vascular depiction, although patent vessels may in certain cases demonstrate a signal void, suggesting occlusion, when this technique is employed (4). Water excitation has been proposed as an alternative to conventional fat suppression (5) such as in the imaging of skeletal muscle. Hauger et al (6) described better image quality in images with water excitation in musculoskeletal imaging. With this technique, only water is excited by using section-selective composite pulses, whereas lipid spins are left in equilibrium and therefore do not produce a signal.
Fig 1.
Example of an MIP reconstruction of TOF images obtained without fat saturation or water excitation, demonstrating interference from bone marrow fat. No special postprocessing was used.
Because water excitation is a relatively new imaging option in standard imaging software, we conducted a feasibility study for intracranial vessel imaging with 10 volunteers to verify the equivalency of image quality between images acquired with and those without water excitation.
Methods
Ten healthy volunteers (seven female, three male) with a mean age of 28 years were studied by using MR imaging. Informed written consent was obtained from all subjects before imaging examinations were performed.
All MR images were acquired by using a 1.5-T MR unit (Sonata, Siemens, Erlangen, Germany). Imaging was performed by using an eight-channel head coil. The imaging protocol consisted of a TOF sequence (FLASH 3D; TR, 40 ms; TE, 4.97 ms; flip angle, 25°; field of view [FOV], 230 mm; effective thickness, 0.83 mm; matrix, 512) first with and then without water excitation. In addition, axial fluid-attenuated inversion recovery (FLAIR) images were obtained from each volunteer to exclude brain diseases. A FLAIR sequence (TE, 108 ms; TR, 9000 ms; flip angle, 25°; FOV, 230 mm; and section thickness, 6 mm) was used.
The water excitation consisted of a 1–1 binomial pair of excitation pulses, each with a flip angle α/2 spaced by 2.39 ms, where α is the desired flip angle of the water spins (5). Both excitation pulses tip the on-resonance magnetization (water spins) toward the x-y plane, whereas spins at the fat frequency are tipped toward the x-y plane by the first pulse and tipped back to the z axis by the second pulse, for zero net excitation.
Two neuroradiologists (I.W., M.F.), who were blinded to the findings, judged images on a scale from 1 to 10, with 1 indicating unacceptable and 10 indicating highly acceptable image quality. Furthermore, images were judged for relevant artifacts. All images were judged by using transverse source images, and MIP reconstructions were also used for judging the results of TOF sequences.
For statistical analysis, a nonparametric paired-data Wilcoxon signed rank test was used with significance defined as P < .05. Doppler ultrasonography was performed as the standard of reference to exclude cerebral artery diseases in all volunteers.
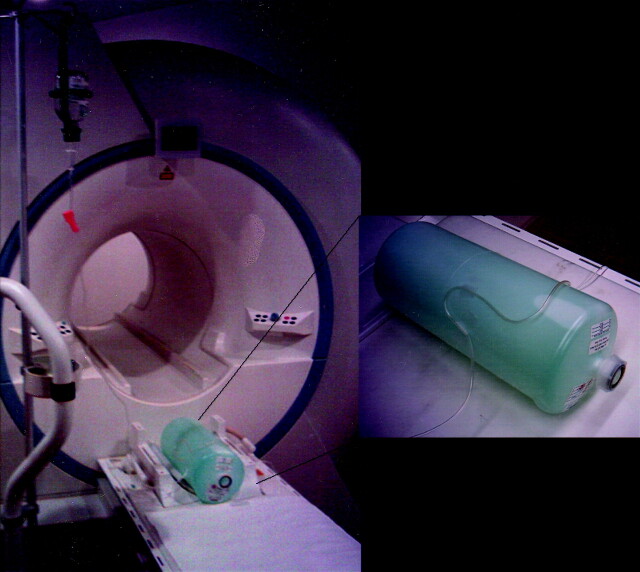
Additional studies by using a phantom with a flow of about 2 mL/s (velocity of about 20 cm/s) were performed with the same TOF sequence parameters first with and then without water excitation. The phantom was created by using a saline infusion tube with flow placed on the surface of a standard MR phantom containing stationary fluid (Fig 2).
Fig 2.
Setup of the flow phantom. The curved path of the tubing with flow was intended to be similar to that of intracranial vessel anatomy. The flow was adjusted to 2 mL/s (20 cm/s).
Results
Doppler ultrasonography confirmed normal cerebral artery flow in all 10 subjects with no indication of disease.
Water excitation did not lead to a significant change in the subjective image quality. Without water excitation, image quality with standard TOF MRA was gradedin the range of 7–9. With water excitation, TOF images were also graded in the range of 7–9. The Wilcoxon test did not reveal any significant difference in image quality grading.
In sequences using water excitation, however, an important pitfall was revealed: apparent carotid artery stenosis was detected in 4/10 and occlusion in 1/10 cases. Furthermore, artifact was detected in the source images as well as in MIP reconstructions. The apparent stenoses, as well as the occlusion, were present throughout the cavernous and supraclinoid segments of the carotid artery.
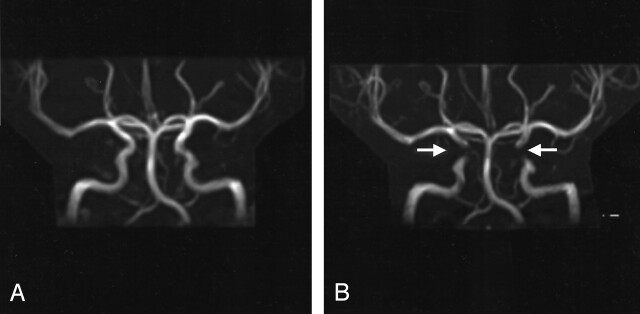
Figure 3A demonstrates the MIP reconstruction of a TOF image obtained without water excitation. The apparent carotid artery occlusion disclosed when using water excitation is demonstrated in Figure 3B.
Fig 3.
MIP reconstructions of two TOF images acquired in the same subject.
A, Normal intracranial arteries acquired with a TOF sequence without water excitation.
B, Carotid artery occlusion is revealed by using a TOF sequence with water excitation.
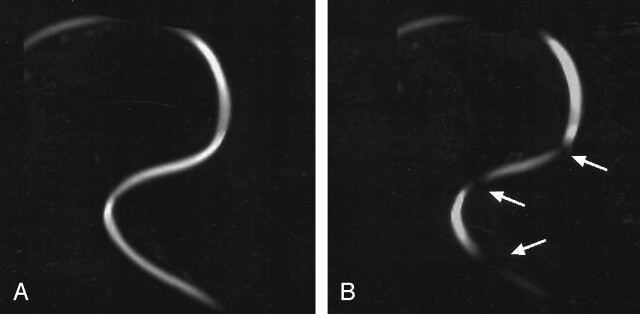
Using the flow phantom, the standard sequence without water excitation again did not lead to any artifact (Fig 4A). The pitfall obtained in vivo could be replicated by using the flow phantom with water excitation sequences (Fig 4B).
Fig 4.
MIP reconstructions of two TOF images acquired in the flow phantom.
A, Continuous flow signal intensity acquired with a standard TOF sequence.
B, Pitfall with partially missing flow signal intensity acquired with a TOF sequence by using water excitation.
Discussion
In TOF MRA, it is known that loss of flow signal can be observed in vessels whose axes lie in the plane of the acquired section. Therefore, apparent vessel occlusion or stenosis is a well-established problem in TOF MRA, but it is easy to identify because of vessel orientation in relation to section acquisition (3). Our results revealed a different kind of apparent carotid artery stenosis that could not be explained by loss of flow signal due to flow direction in the acquired sections. These artifacts were located in vessel segments that had a nearly vertical flow direction, almost orthogonal to the section plane (see Fig 3B).
Similar artifacts were described by Mirowitz et al (4) when using conventional fat saturation in cerebral TOF MRA. He argues that these artifacts could be induced by an imperfect section profile of the presaturation pulse or unintended saturation of flowing spins. Therefore, fat suppression is not recommended for standard TOF MRA.
Without this suppression, however, the high signal intensity of bone marrow fat can interfere with vessel depiction, especially in MIP reconstructions (3) (Fig 1). On the other hand, because specialized postprocessing and image cutting can reduce the interference nearly completely and because radiologists base their diagnosis primarily on the source images rather than the MIP reconstructions, it is considered more prudent to avoid strong flow artifacts from fat saturation and accept the signal intensity from bone marrow.
Because the water excitation method is inherently different from the application of fat saturation, which uses a presaturation pulse, there seems to be a different mechanism producing a similar artifact. Several experiments not documented here were performed to test various explanations for the artifact formation, but without a definitive explanation emerging. It is interesting to note that the artifact generally occurred in vessel bends, where flow might be quite complex, with a distribution of flow velocities across the vessel lumen skewed to the outer radius of the bend. This observation was confirmed in the phantom experiments.
Conclusion
Because our results indicate that TOF MRA does not necessitate the water excitation pulse for satisfactory image interpretation while using source images and addition MIP reconstruction, we would currently warn against its use. Water excitation can even lead to an important pitfall in the interpretation of the images and incorrect diagnosis of diseases in the intracranial arteries in both source images and MIP reconstructions. If this type of water excitation is nevertheless used in cerebral TOF MRA, awareness of this diagnostic pitfall is important so that serious misdiagnosis does not occur.
References
- 1.Horiguchi J, Mori H, Kiso T. Increasing resolution of cerebral small arteries in 3D TOF MR angiography: efficacy of nitroglycerin-MRA (NTG-MRA) in patients with neurovascular disease. Nippon Igaku Hoshasen Gakkai Zasshi 1996;56:708–711 [PubMed] [Google Scholar]
- 2.Wiesmann M, Yousry I, Seelos KC, Yousry TA. Identification and anatomic description of the anterior choroidal artery by use of 3D-TOF source and 3D-CISS MR imaging. AJNR Am J Neuroradiol 2001;22:305–310 [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson CM, Saloner D, Tsuruda JS, et al. Artifacts in maximum-intensity-projection display of MR angiograms. AJR Am J Roentgenol 1990;154:623–629 [DOI] [PubMed] [Google Scholar]
- 4.Mirowitz SA. Apparent vascular occlusion on cranial TOF MRA with peripheral presaturation technique. J Comput Assist Tomogr 1993;17:927–931 [DOI] [PubMed] [Google Scholar]
- 5.Thomasson D, Purdy D, Finn JP. Phase-modulated binomial RF pulses for fast spectrally-selective musculoskeletal imaging. Magn Reson Med 1996;35:563–568 [DOI] [PubMed] [Google Scholar]
- 6.Hauger O, Dumont E, Chateil JF, et al. Water excitation as an alternative to fat saturation in MR imaging: preliminary results in musculoskeletal imaging. Radiology 2002;224:657–663 [DOI] [PubMed] [Google Scholar]