Abstract
BACKGROUND AND PURPOSE: Dynamic perfusion CT (PCT) with deconvolution produces maps of time-to-peak (TTP), mean transit time (MTT), regional cerebral blood flow (rCBF), and regional cerebral blood volume (rCBV), with a computerized automated map of the infarct and penumbra. We determined the accuracy of these maps in patients with suspected acute hemispheric stroke.
METHODS: Forty-six patients underwent nonenhanced CT and dynamic PCT, with follow-up CT or MR imaging. Two observers reviewed the nonenhanced studies for signs of stroke and read the PCT maps for TTP, MTT, rCBF, and rCBV abnormalities. Sensitivity, specificity, accuracy, and interobserver agreement were compared (Wilcoxon tests). Nonenhanced CT and PCT data were reviewed for stroke extent according to previously reported methods. Sensitivity, specificity, and accuracy of the computerized maps in detecting ischemia and its extent were determined.
RESULTS: Compared with nonenhanced CT, PCT maps were significantly more accurate in detecting stroke (75.7–86.0% vs. 66.2%; P < .01), MTT maps were significantly more sensitive (77.6% vs. 69.2%; P < .01), and rCBF and rCBV maps were significantly more specific (90.9% and 92.7%, respectively, vs. 65.0%; P < .01). Regarding stroke extent, PCT maps were significantly more sensitive than nonenhanced CT (up to 94.4% vs. 42.9%; P < .01) and had higher interobserver agreement (up to 0.763). For the computerized map, sensitivity, specificity, and accuracy, respectively, were 68.2%, 92.3%, and 88.1% in detecting ischemia and 72.2%, 91.8%, and 87.9% in showing the extent.
CONCLUSION: Dynamic PCT maps are more accurate than nonenhanced CT in detecting hemispheric strokes. Despite limited spatial coverage, PCT is highly reliable to assess the stroke extent.
Perfusion CT (PCT) techniques combined with CT angiography (CTA) provide a comprehensive noninvasive survey of the site of arterial occlusion and the resulting ischemia and infarction (1). Two categories of PCT techniques are currently in practice: whole-brain PCT and section-selective dynamic PCT. Whole-brain PCT provides information related to cerebral blood volume (CBV) and the infarct core (2, 3), with reported sensitivity, specificity, and accuracy of 90.5%, 89.5% and 90.0%, respectively (4). It does not, however, permit assessment of the ischemic penumbra (5), which is the target of acute stroke treatment.
Section-selective dynamic PCT involves the acquisition of sequential CT data from a few sections in a cine mode during an intravenous bolus of iodinated contrast material. Dynamic PCT data can be analyzed according to two distinct mathematical models: the maximal slope model and the central volume principle (6). The maximal slope model has a sensitivity and specificity of 93% and 98%, respectively, as reported in small series of patients (7, 8), although it has the major drawback of not being quantitative (6). The central volume principle, which relies on a mathematical operation called deconvolution, assesses cerebral blood flow (CBF), CBV, and mean transit time (MTT) in a robust and reproducible way; it also has the advantage of being quantitative (9–12). The computerized automated map of infarct and penumbra (13, 14) is correlated with findings on MR-based diffusion-weighted (DW) imaging and perfusion-weighted imaging, both in the acute phase (13) and on follow-up (14).
In clinical practice, PCT maps are interpreted in conjunction with nonenhanced CT and CTA images in patients with suspected stroke. However, the accuracy of the maps obtained during dynamic PCT with deconvolution has not been fully assessed. Which of the maps is most reliable for the detection of stroke? What are the advantages of each of the maps compared with the others?
Whatever PCT map is considered, the limited spatial coverage of dynamic PCT might represent a major drawback and jeopardize its sensitivity. With multidetector-row CT technology, the spatial coverage of dynamic PCT can reach 20–24 mm for each bolus of contrast material. When we consider the risk of nephrotoxicity and the amount of contrast material that must be reserved for subsequent CTA (15), a maximum of two 40–50-mL boluses can be used; therefore, total coverage up to 40–48 mm reached. To increase coverage with CT perfusion during a single bolus of contrast agent, certain technical manipulations, such as the toggling-table technique, have been proposed (16). These can potentially double the spatial coverage afforded by PCT.
In this study, we assessed the different maps derived from dynamic PCT with deconvolution in the setting of suspected acute hemispheric stroke. Our goal was to determine the sensitivity, specificity, and accuracy of the maps of TTP, MTT, regional CBF (rCBF), and regional CBV (rCBV), as well as the computerized automated map, for demonstrating acute brain ischemia and its extent. To assess the potential impact of the limited spatial coverage of PCT, we compared findings on the four 10-mm sections included in our PCT protocol to the findings on whole-brain nonenhanced CT scans.
Methods
Design
Imaging data obtained as part of standard clinical stroke care at our institution were retrospectively reviewed with the approval of our institutional review board. At our institution, patients with suspected acute stroke and no notable renal insufficiency or allergy to contrast agent undergo the following imaging protocol: nonenhanced CT, PCT at two cross-sectional positions, CTA of the cervical and intracranial vessels, and contrast-enhanced cerebral CT (15). The acquisition parameters for the PCT series are described later.
Nonenhanced CT and PCT data were included in this study if these conditions were met: 1) The patient presented to the emergency department with signs and symptoms suggestive of hemispheric stroke (e.g., hemisensory loss, hemiparesis, aphasia, or homonymous visual field abnormality) of 12 hours’ duration or less, 2) there was no evidence of intracerebral hemorrhage, and 3) the patient underwent follow-up CT or MR imaging to confirm or rule out ischemic hemispheric stroke. Patients who underwent thrombolysis were excluded.
Imaging Protocol
PCT consisted of a 45-second series with 45 gantry rotations performed in cine mode during the intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of one image per second, resulting in a series of 45 images for each assessed section. Two successive PCT series were performed after nonenhanced CT and before CTA. The two PCT series were separated by 5 minutes.
Multidetector-row CT technology permitted the assessment of two adjacent 10-mm-thick sections for each series. Two 10-mm-thick sections were preferred to four adjacent 5-mm-thick sections per location to maximize the signal-to-noise ratio without increasing the dose to the patient. The two sections of the first PCT series were at the level of the third ventricle and the basal ganglia, positioned above the orbits to protect the lenses. The second PCT series was selected at a level 3.5 cm rostral to the first section of the first series.
For each series, a 40-mL bolus of iohexol (Omnipaque, iodine 300 mg/mL; Amersham Health, Princeton, NJ) was administered into an antecubital vein by using a power injector at an injection rate of 4 mL/s. The acquisition parameters were 80 kVp and 120 mAs. CT scanning was initiated 7 seconds after the start of the injection. The time delay before contrast material reached the brain parenchyma allowed the acquisition of nonenhanced baseline images.
Data Processing
PCT data were analyzed by using PCT prototype software (Philips Medical Systems, Best, the Netherlands). This software relies on the central volume principle, which is the most accurate for low injection rates of iodinated contrast material (6). After motion correction and noise reduction are done by using an anisotropic, edge-preserving spatial filter, the software applies curve fitting by a least-mean-squares method to obtain mathematical descriptions of the time-attenuation curves. Then, it applies a closed-form (noniterative) deconvolution to calculate the MTT map (17). The deconvolution operation requires a reference arterial input function, which selected by the PCT software in a region of interest that the user draws around the anterior cerebral artery (ACA). The rCBV map is calculated from the areas under the time-enhancement curves (18). A simple equation combining rCBV and MTT values allows the calculation of rCBF: rCBF = rCBV/MTT (17). Finally, the software calculates an automated computerized map of the penumbra and infarct by using rCBF and rCBV thresholds, according to a method previously described and validated (13, 14).
Data Analysis
Two observers (a neuroradiologist [W.P.D.] and a stroke neurologist [W.S.S.]) reviewed each of the 46 nonenhanced CT studies obtained on admission. They evaluated the images for conventional signs of stroke: hemispheric hypoattenuation or swelling, loss of the cortical ribbon (including the insular ribbon), hypoattenuation in the basal ganglia, and the attenuated artery sign. They also assess the studies according to the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) (19) and Alberta Stroke Program Early CT Score (ASPECTS) systems (20). For the ATLANTIS system, the reviewers evaluated hypoattenuation in four areas: frontal, temporal, parietal, and basal ganglia and insular cortex. If two or more areas showed hypoattenuation, the patient was considered to have a stroke involving more than one third of the middle cerebral artery (MCA) territory (19). For the ASPECTS system, the reviewers evaluated hypoattenuation in 10 areas: caudate, lenticulate, internal capsule, insula, M1, M2, M3, M4, M5, and M6. Their assessments lead to a score ranging from 10 (no areas with hypoattenuation) to 0 (10 areas with hypoattenuation). If the score was 7 or less, the patient was considered to have a stroke involving more than one third of the MCA territory (20). Finally, the reviewers assessed hypoattenuation in the arterial territories of the ACA and posterior cerebral artery (PCA).
In separate review sessions, the same reviewers reviewed the rCBV, rCBF, MTT, and time-to-peak (TTP) maps for abnormally low rCBV, low rCBF, high MTT, and high TTP values in the four areas defined in the ATLANTIS method, the 10 areas defined in the ASPECTS method, and the ACA and PCA territories. If two or more areas from the ATLANTIS method or if three or more areas from the ASPECTS method showed abnormal values, the patient was considered to have a stroke involving more than one third of the MCA territory, as shown on the corresponding PCT map. Special attention was paid to the blinding of patient data. Moreover, reviews of the nonenhanced CT scans and rCBV, rCBF, MTT, and TTP maps were performed successively, at 2-week intervals. The reviewers performed the readings independently of each other and were blinded to the other’s opinions, as well as the results of the formal, dictated evaluation of these examinations. The reviewers were informed of only the body side of the signs and symptoms to reproduce the emergency setting as closely as possible.
The reviewers’ assessment of the PCT maps relied on their subjective visual evaluation and involved a comparison of the presumed pathologic hemisphere with the presumed healthy one. The data were also subjected to automated analysis by PCT prototype software (Philips Medical Systems). As mentioned before, this software automatically defines a computerized map of the infarct and penumbra based on thresholds levels of rCBF and rCBV. This computerized automated map was assessed for possible penumbra or infarct in the four ATLANTIS areas, the 10 ASPECTS areas, and the ACA and PCA territories.
Statistical Analysis
In each patient, follow-up CT or MR images were reviewed according to the scheme just described. The results were considered the criterion standard for the calculation of the sensitivity, specificity, and accuracy of hypoattenuation on nonenhanced CT and of rCBV, rCBF, MTT, and TTP abnormalities on PCT. Sensitivity, specificity, and accuracy were calculated for the detection of stroke and also for the assessment of the extent (more or less than one third of the MCA territory).
For each conventional nonenhanced CT sign (erased cortex, erased insula, erased basal nuclei, attenuated artery sign), the sensitivity, specificity, and accuracy were determined depending on whether a final diagnosis of stroke was confirmed or not. Sensitivity, specificity, and accuracy were also calculated for all the conventional nonenhanced CT signs considered together (e.g., the study was considered positive if any of the previously listed signs were present). These global values for nonenhanced CT were used as a reference in Wilcoxon (Mann-Whitney) tests to determine whether the different PCT maps were superior to nonenhanced CT. The significance level was set at .01 to account for the multiple sampling (Bonferroni correction).
Cohen κ coefficients were calculated to determine the interobserver agreement (between the neuroradiologist and the stroke neurologist). These were determined for both the presence and the extent of abnormalities on nonenhanced CT and PCT. A κ value of 0.20 or less implied poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, excellent agreement (21).
Results
Patient Characteristics
Between January and June 2003, 147 patients presented to the emergency department with strokelike symptoms and underwent our stroke CT protocol. Among them, we identified 46 who fulfilled the inclusion criteria. Thirty-one were male and 15 female, with a median age of 64 years (range, 28–94 years). Their symptoms and signs of stroke were left sided in 28 and right sided in 18. The median time between stroke onset and performance of the admission CT protocol was 5.5 hours, with a range of 1.0–12.0 hours. Twenty-nine patients underwent follow-up CT (median, 5 days; range, 3–18 days), and 17 underwent follow-up MR imaging (median, 5 days; range, 2–8 days), which included both DW imaging and fluid-attenuated inversion recovery series. Most patients did not undergo CTA or MR angiography during their follow-up examination. Final diagnoses were acute ischemic stroke in 26 (23 nonlacunar and three lacunar), transient ischemic attack (TIA) in 14, and nonstroke conditions in six (seizures in four, migraine in two). The location of the strokes is summarized in Table 1. Seven patients were also had strokes before the event precipitating the stroke CT protocol.
TABLE 1:
Territorial location of strokes on follow-up
| Scoring System | Number (n = 26) |
|---|---|
| Major arterial territory | |
| ACA | 2 (7.7) |
| MCA | 17 (65.4) |
| PCA | 11 (42.3) |
| ATLANTIS | |
| Frontal | 12 (46.2) |
| Parietal | 8 (30.8) |
| Temporal | 8 (30.8) |
| Basal ganglia | 9 (34.6) |
| ASPECTS | |
| Caudate | 4 (15.4) |
| Lenticulate | 8 (30.8) |
| Internal capsule | 7 (26.9) |
| Insula | 10 (38.5) |
| M1 | 5 (19.2) |
| M2 | 10 (38.5) |
| M3 | 7 (26.9) |
| M4 | 7 (26.9) |
| M5 | 14 (53.8) |
| M6 | 5 (19.2) |
Note.—Data in parentheses are percentages. The MCA territory was involved in about two-thirds of the 26 stroke patients. Patients may have had more than one infarcted area.
Nonenhanced CT and PCT in Detecting Stroke
The sensitivity, specificity, accuracy, and interobserver agreement of nonenhanced CT and PCT in the assessment of stroke are summarized in Table 2. The accuracy of PCT was significantly greater than that of nonenhanced CT (75.7–86% vs. 66.2%; P < .01) in the detection of stroke. TTP and MTT maps were significantly more sensitive than nonenhanced CT (76.5% and 77.6%, respectively, vs. 69.2%; P < .01), with a higher interobserver agreement for MTT maps (i.e., 0.618). rCBF and rCBV were significantly more specific then nonenhanced CT (90.9% and 92.7%, respectively, vs. 65.0%; P < .01). On the other hand, interobserver agreement only tended to be better with PCT than with nonenhanced CT. The automated method for delineating brain ischemia had sensitivity, specificity, and accuracy similar to those resulting from the observers’ review; interobserver-agreement issues did not apply, as since it was automated method.
TABLE 2:
Sensitivity, specificity, accuracy, and interobserver agreement in detecting stroke
| Imaging Study | True-Positive | False-Positive | False-Negative | True-Negative | Sensitivity (%) | Specificity (%) | Accuracy (%) | κ |
|---|---|---|---|---|---|---|---|---|
| Nonenhanced CT | ||||||||
| Any sign | 18 | 23 | 8 | 43 | 69.2 | 65.0 | 66.2 | 0.502 |
| Lost cortical ribbon | 11 | 5 | 15 | 61 | 43.4 | 92.3 | 78.5 | 0.528 |
| Lost insular ribbon | 7 | 3 | 19 | 63 | 26.9 | 95.0 | 75.8 | 0.591 |
| Basal ganglia hypodensity | 15 | 10 | 12 | 56 | 55.8 | 85.0 | 76.7 | 0.478 |
| Dense artery | 6 | 10 | 20 | 56 | 23.1 | 85.0 | 67.5 | 0.462 |
| ATLANTIS/ASPECTS hypoattenuations | 115 | 77 | 139 | 1141 | 45.2 | 93.7 | 85.3 | 0.594 |
| PCT | ||||||||
| TTP review | 194 | 298 | 60 | 920 | 76.5* | 75.6 | 75.7* | 0.526 |
| MTT review | 197 | 288 | 57 | 930 | 77.6* | 76.3 | 76.5* | 0.618 |
| rCBF review | 158 | 111 | 96 | 1107 | 62.2 | 90.9* | 86.0* | 0.516 |
| rCBV review | 128 | 89 | 126 | 1129 | 50.5 | 92.7* | 85.4* | 0.505 |
| Computerized automated method | 173 | 94 | 81 | 1124 | 68.2 | 92.3* | 88.1* | NA |
Note.—Follow-up CT or MR imaging was the criterion standard. Globally, accuracy was significantly greater with PCT than with nonenhanced CT: TTP and MTT maps are significantly more sensitive, whereas rCBF and rCBV maps were significantly more specific. NA indicates not applicable.
Significantly superior to nonenhanced CT (any sign).
False-Negative and False-Positive Findings
False-negative findings on PCT were related mainly to a lack of spatial coverage. PCT failed to completely reveal a nonlacunar stroke in only one of the 23 patients. In three cases, follow-up imaging demonstrated acute lacunar strokes that were too small to be detected on PCT maps. The other false-negative cases were false-negative areas in patients with true-positive disease (i.e., in each of these patients) PCT demonstrated most but not all ischemic areas. In two cases, hyperemia (high rCBF and rCBV, low MTT and TTP) rather than oligemia was recorded in a territory in which follow-up imaging showed infarct; this led to false-negative results according to the assessment criteria used. This pattern was also identified in three patients with a final diagnosis of TIA; this finding will need to be accounted for in future grading systems. Finally, in four patients, PCT maps were degraded by motion artifacts that compromised the detection of oligemia in some areas; this also led to false-negative results.
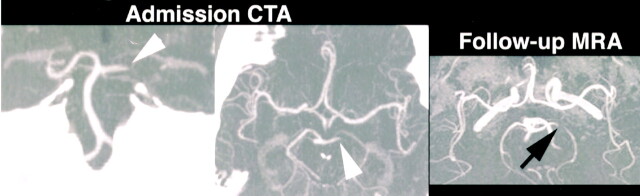
One case of arterial recanalization was also documented (Fig 1). In this case, the rCBF, MTT, and TTP maps were abnormal in some areas that were negative for stroke on follow-up examination. The computerized automated map described these areas as penumbra. False-positive results with PCT also occurred in three cases of TIA, in which TTP and MTT maps suggested ischemia (Fig 2). Remote infarctions also led to false-positive results, as they could be distinguished from acute strokes only when PCT maps were interpreted side by side with the corresponding nonenhanced CT.
Fig 1.
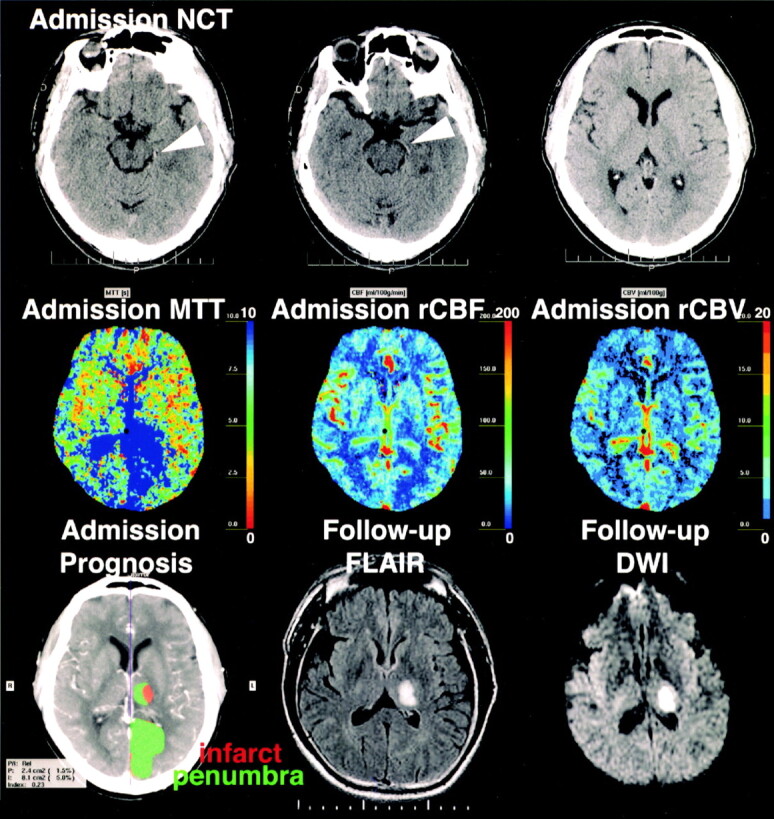
55-year-old man with right homonymous hemianopsia and right-body hemisensory loss. Admission nonenhanced CT 6 hours after symptom onset was unremarkable (both reviewers), except for an attenuated left PCA (arrowheads, one reviewer). PCT shows prolonged MTT and reduced rCBF in the left PCA territory. rCBVs are reduced only in a small part of the left thalamus but are increased in the rest of the PCA territory. The areas correspond to infarct and penumbra on the computerized automated map. Figure and legend continues.
Fig 2.
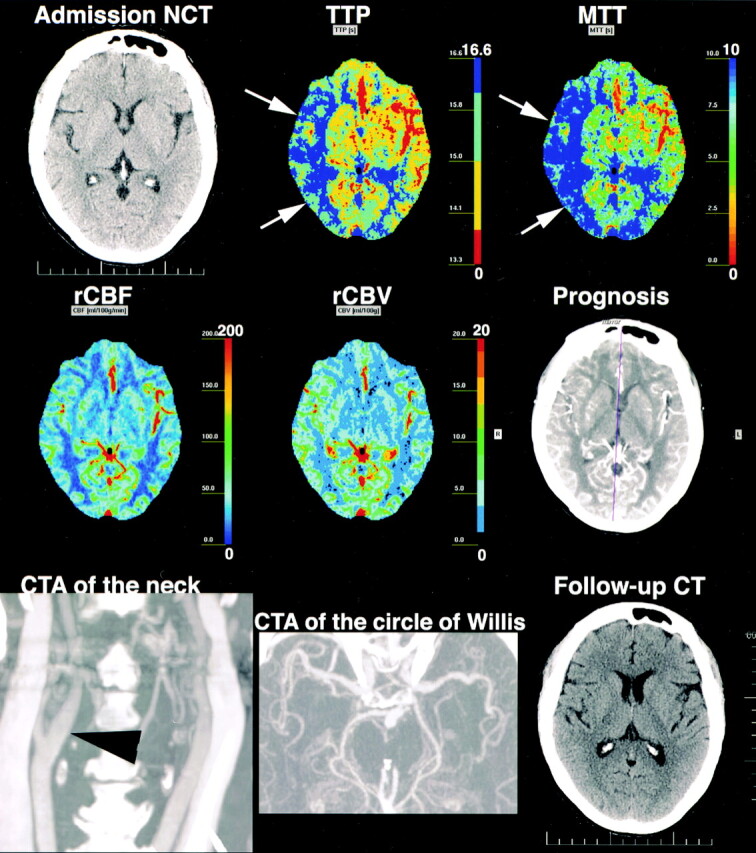
77-year-old woman with acute-onset left hemiparesis. Admission nonenhanced CT 2 hours after onset is normal. TTP and MTT are prolonged in the right superficial MCA territory (arrows). rCBFs are normal, but rCBVs are higher than contralateral values. TTP and MTT changes are not explained by any vascular abnormality; right carotid bifurcation (arrowhead) and intracranial arteries are normal. Final diagnosis was TIA. Follow-up CT 14 days later was normal. TTP and MTT changes were regarded as false-positive and most likely related to luxury perfusion due to TIA.
Nonenhanced CT and PCT in Assessing Stroke Extent
In assessing the extent of stroke (more or less than one third of the MCA territory), PCT is more sensitive (up to 94.4%), specific (up to 92.1%) and accurate (up to 87.9%) than nonenhanced CT (62.8%). PCT also had significantly increased interobserver agreement. This finding was true with both the ASPECTS (Table 3) and the ATLANTIS scoring systems.
TABLE 3:
Sensitivity, specificity, accuracy, and interobserver agreement in assessing stroke extent by ASPECTS score (>  ASPECTS)
ASPECTS)
| Imaging Study | ASPECTS Score | Sensitivity (%) | Specificity (%) | Accuracy (%) | κ |
|---|---|---|---|---|---|
| Nonenhanced CT | 8.4 ± 1.9 | 42.9 | 67.6 | 62.8 | 0.446 |
| PCT | |||||
| TTP review | 6.9 ± 2.5 | 87.5* | 68.4 | 72.2 | 0.716* |
| MTT review | 7.2 ± 2.3 | 94.4* | 64.1 | 70.0 | 0.763* |
| rCBF review | 7.4 ± 2.3 | 85.7* | 87.2* | 86.9* | 0.691* |
| rCBV review | 7.8 ± 2.1 | 62.5* | 92.1* | 86.3* | 0.669* |
| Computerized automated method | 7.7 ± 2.3 | 72.2* | 91.8* | 87.9* | NA |
| Follow-up | 7.4 ± 2.1 | NA | NA | NA | NA |
Note.—PCT was globally more sensitive, specific, and accurate than nonenhanced CT in correctly classifying large strokes, with significantly increased interobserver agreement. NA indicates not applicable.
Significantly superior to nonenhanced CT.
Discussion
We found that dynamic PCT maps were significantly more accurate than nonenhanced CT in the detection of stroke (75.7–88.1% vs. 66.2%; P < .01) in patients with hemispheric stroke symptoms of less than 12 hours ’ duration. MTT and TTP maps were significantly more sensitive than nonenhanced CT (77.6% and 76.5%, respectively, vs. 69.2%; P < .01), with higher interobserver agreement for MTT maps (0.618 for MTT vs. 0.526 for TTP). On the other hand, rCBF and rCBV maps were significantly more specific (90.9% and 92.7%, respectively). Sensitivity, specificity, accuracy, and interobserver agreement for nonenhanced CT were consistent with those previously reported (22–25). Conventional nonenhanced CT signs (hemispheric hypoattenuation, loss of the cortical ribbon, hypoattenuation in the basal ganglia, attenuated artery sign) were specific (85.0–95.0%) but not sensitive (23.1–55.8%), since most of these signs occur relatively late in the course of acute brain ischemia (26, 27). This observation was consistent with their occurrence, which was lower than that of PCT abnormalities. The reviewers identified 115 true-positive hypoattenuating areas on the nonenhanced CT, while 197 true-positive abnormal areas were identified on MTT maps.
False-negative PCT results were related mainly to a lack of spatial coverage, as has been reported for other dynamic PCT techniques (28, 29). However, with PCT, a nonlacunar stroke was missed in only one of 23 patients. We also identified false-negative areas occurring in patients with true-positive disease (i.e., PCT demonstrated most ischemic areas but missed some). Several lacunar strokes were not identified on PCT because of a lack of spatial resolution. In two patients, we identified a pattern that was responsible for false-negative results based on the classification scheme used in our study. This pattern consisted of hyperemia (high rCBF and rCBV, low MTT and TTP) rather than oligemia in a territory in which follow-up imaging subsequently showed infarct. This pattern was likely related to the so-called luxury perfusion and should be accounted for in future studies.
Most of the false-positive PCT results were associated with TIA. In TIA, MTT is prolonged, but rCBV is also increased, presumably because of sufficient circulation and cerebral vascular autoregulation, with subsequent preservation of rCBF. In a sense, however, these are only false-positive for predicting infarction, as the patient presumably does have ischemia but insufficient tissue compromise to lead to infarction. False-positive PCT results also occurred in cases of remote infarctions, which typically have the same PCT characteristics as acute ones (prolonged TTP and MTT, lowered rCBF and rCBV). Remote infarctions are easily distinguished from acute ones (less than 12 hours) on the basis of nonenhanced CT. However, the reviewers in this study, evaluated nonenhanced CT and PCT maps in different sessions and not together, increasing the false-positive PCT results. The impact of combined interpretation of nonenhanced CT and PCT studies was not analyzed, but is expected to reduce such false-positive results.
The clinical interpretation of PCT data should include evaluation of a sensitive map to screen acute patients for brain ischemia and also a specific map to diagnose infarction. MTT and TTP maps are both highly sensitive for detecting ischemia. TTP maps, however, had lower interobserver agreement and are sensitive to extracranial or intracranial vascular stenosis, independent of brain ischemia. Therefore, MTT maps are more useful in screening for ischemia, with the caveat that they are abnormal not only in strokes but also in TIA. rCBF and rCBV maps are the most specific PCT maps, and their combined interpretation in the computerized automated method had an accuracy of 88.1%. Our study design was such that it was impossible to systematically assess the influence of recanalization or persistent arterial occlusion—and also the resolution of penumbra or the evolution of infarction over penumbra—on the accuracy of the PCT maps, since we did not have follow-up CTA or MR angiographic studies in most patients.
Our proposed interpretation scheme based on sensitive MTT maps and specific rCBF and rCBV maps must still be validated in a prospective study with respect to its effect on patient care; this is also the case for the computerized automated maps. The reviewers did not undertake a combined interpretation of the different PCT maps together so that we could explore the value of interpreting each map in isolation. With the results of this study, we expect the sensitivity and specificity of PCT—coupled with nonenhanced CT findings—to be substantially higher if reviewers use MTT to identify ischemia and rCBF and rCBV maps to confirm ischemia in regions with prolonged MTT. Additionally, the incorporation of CTA results might also enhance this accuracy; this is the subject of further study.
Another important factor in the diagnosis and treatment of stroke is the assessment of infarction extent, as this is an important criterion in the selection of patients for acute treatment (30, 31). Our study demonstrated that PCT was significantly more sensitive (up to 94.4%), more specific (up to 93.9%), and more accurate (up to 87.9%) than nonenhanced CT in the detection of stroke involving more than one third of the MCA territory. Interobserver agreement for PCT (up to 0.763) was substantial and significantly higher than that of nonenhanced CT (i.e., 0.446). This was true whether the ATLANTIS or the ASPECTS-derived scores were used.
This finding has important implications on stroke management. Indeed, the 37.0–42.9% sensitivity of nonenhanced CT for strokes more extensive one third of the MCA territory suggests that five to eight (10.9–17.4%) of our 46 patients with large infarcts on follow-up would have undergone unindicated aggressive treatment or invasive diagnostic procedures (e.g., angiography) if the volume of infarction at baseline was based on nonenhanced CT alone. With PCT, the percentage of patients referred for aggressive acute stroke treatment, which is not indicated on the basis of the one-third MCA guidelines, could be theoretically lowered to 2.2%. Thus, PCT constitutes an important tool for the stratification of patients for acute stroke treatment, and it may potentially result in substantial cost savings and reductions in morbidity. However, whether the European Cooperative Acute Stroke Study (ECASS) and National Institute of Neurological Disorders and Stroke (NINDS) criteria regarding nonenhanced CT findings can be directly transferred to PCT is unclear. We are likely dealing with a new tool that defines the volume of brain at risk more precisely that previous methods, and we postulate that PCT parameters may help in deciding who is at risk for hemorrhage and who is likely to benefit from aggressive intervention.
We did not directly compare the performances of PCT and DW imaging in the early detection of brain ischemia. For PCT, we found an overall sensitivity of 77.6%, which can be compared with the 86–94% reported for DW imaging (32–34). Interobserver agreement for PCT (0.505–0.618) was similar to the 0.6 reported for DW imaging (35, 36). Our study also showed that PCT has high sensitivity (up to 94.4%), specificity (up to 93.9%), and accuracy (up to 87.9%), and interobserver agreement (up to 0.716) in the depiction of stroke extent. However, a direct comparison with acute DW imaging in this feature remains to be performed.
We enrolled only patients with suspected hemispheric stroke, limiting the number of patients with lacunar or other small strokes and those with strokes in the posterior fossa. PCT technique is limited in the diagnosis of lacunae and strokes in the posterior fossa because of limited spatial resolution and artifacts in this fossa. Additionally, imaging of the posterior fossa with PCT results in a substantial radiation dose to the lenses.
Another limitation of our study is the requirement of follow-up scanning for inclusion in the study. This criterion excluded a large fraction of those with TIA and might have positively influenced the accuracy measures by lowering the number of nonstroke patients. However, because our goal was to calculate the sensitivity, specificity, and accuracy of nonenhanced CT and PCT maps—and ultimately to compare nonenhanced CT and PCT techniques—we needed a criterion standard other than nonenhanced CT or PCT; therefore, a follow-up examination was required.
Conclusion
Deconvolution-based dynamic PCT maps are more accurate than nonenhanced CT scans in the depiction of acute hemispheric stroke. MTT maps are the most sensitive, and rCBF and rCBV maps the most specific. Moreover, PCT more accurately depicts the extent of stroke; this advantage has implications on clinical decision making. Despite its limited spatial coverage, PCT can likely be used to stratify patients for acute stroke treatment. An interpretation scheme based on sensitive MTT maps and specific rCBF and rCBV maps must be validated in a prospective study with respect to its effect on patient care.
Fig 1.
Continued.—Admission CTA shows an occluded left P1 segment, which had recanalized but remained focally stenotic on follow-up MR angiography 3 days later. Follow-up DW image shows completed stroke in the predicted left thalamic infarct core. The penumbra did not infarct, most likely because of early recanalization; the MTT and rCBF abnormalities were however, categorized as false-positive since delayed MR imaging was selected as the criterion standard.
Footnotes
Supported by grants from the Swiss Society of Radiology, the Société Académique Vaudoise, and the SICPA Foundation.
References
- 1.Latchaw RE, Yonas H, Hunter GJ, et al. Council on Cardiovascular Radiology of the American Heart Association: guidelines and recommendations for perfusion imaging in cerebral ischemia. Stroke 2003;34:1084–1104 [DOI] [PubMed] [Google Scholar]
- 2.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 2001;32:2021–2027 [DOI] [PubMed] [Google Scholar]
- 3.Hamberg LM, Hunter GJ, Maynard KI, et al. Functional CT perfusion imaging in predicting the extent of cerebral infarction from a 3-hour middle cerebral arterial occlusion in a primate stroke model. AJNR Am J Neuroradiol 2002;23:1013–1021 [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter GJ, Silvennoinen HM, Hamberg LM, et al. Whole-brain CT perfusion measurement of perfused cerebral blood volume in acute ischemic stroke: probability curve for regional infarction. Radiology 2003;227:725–730 [DOI] [PubMed] [Google Scholar]
- 5.Wintermark M, Bogousslavsky J. Imaging of acute ischemic brain injury: the return of computed tomography. Curr Opin Neurol 2003;16:59–63 [DOI] [PubMed] [Google Scholar]
- 6.Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 2001;11:1220–1230 [DOI] [PubMed] [Google Scholar]
- 7.Mayer TE, Hamann GF, Baranczyk J, et al. Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol 2000;21:1441–1449 [PMC free article] [PubMed] [Google Scholar]
- 8.Reichenbach JR, Rother J, Jonetz-Mentzel L, et al. Acute stroke evaluated by time-to-peak mapping during initial and early follow-up perfusion CT studies. AJNR Am J Neuroradiol 1999;20:1842–1850 [PMC free article] [PubMed] [Google Scholar]
- 9.Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R. Simultaneous measurements of regional cerebral blood flows by perfusion-CT and stable xenon-CT: a validation study. AJNR Am J Neuroradiol 2001;22:905–914 [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa M, Kashiwagi S, Matsunaga N, Suzuki M, Kishimoto K, Shirao S. Evaluation of cerebral perfusion parameters measured by perfusion CT in chronic cerebral ischemia: comparison with Xenon CT. J Comput Assist Tomogr 2002;26:272–278 [DOI] [PubMed] [Google Scholar]
- 11.Kudo K, Terae S, Katoh C, Oka M, Shiga T, Tamaki N, Miyasaka K. Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H215O positron emission tomography. AJNR Am J Neuroradiol 2003;24:419–426 [PMC free article] [PubMed] [Google Scholar]
- 12.Gillard JH, Antoun NM, Burnet NG, Pickard JD. Reproducibility of quantitative CT perfusion imaging. Br J Radiol 2001;74:552–555 [DOI] [PubMed] [Google Scholar]
- 13.Wintermark M, Reichhart M, Cuisenaire O, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 2002;33:2025–2031 [DOI] [PubMed] [Google Scholar]
- 14.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–432 [DOI] [PubMed] [Google Scholar]
- 15.Smith WS, Roberts HC, Chuang NA, et al. Safety and feasibility of a CT protocol for acute stroke: combined CT, CT angiograph, and CT perfusion imaging in 53 consecutive patients. AJNR Am J Neuroradiol 2003;24:688–690 [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts HC, Roberts TP, Smith WS, Lee TJ, Fischbein NJ, Dillon WP. Multisection dynamic CT perfusion for acute cerebral ischemia: the “toggling-table” technique. AJNR Am J Neuroradiol 2001;22:1077–1080 [PMC free article] [PubMed] [Google Scholar]
- 17.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 1983;8:94–99 [DOI] [PubMed] [Google Scholar]
- 18.Ladurner G, Zilkha E, Iliff D, du Boulay GH, Marshall J. Measurement of regional cerebral blood volume by computerized axial tomography. J Neurol Neurosurg Psychiatry 1976;39:152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalafut MA, Schriger DL, Saver JL, Starkman S. Detection of early CT signs of > 1/3 middle cerebral artery infarctions: interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke 2000;31:1667–1671 [DOI] [PubMed] [Google Scholar]
- 20.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–1542 [PMC free article] [PubMed] [Google Scholar]
- 21.Kundel HL, Polansky M. Measurement of observer agreement. Radiology 2003;228:303–308 [DOI] [PubMed] [Google Scholar]
- 22.von Kummer R, Bourquain H, Bastianello S, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 2001;219:95–100 [DOI] [PubMed] [Google Scholar]
- 23.von Kummer R, Holle R, Gizyska U, et al. Interobserver agreement in assessing early CT signs of middle cerebral artery infarction. AJNR Am J Neuroradiol 1996;17:1743–1748 [PMC free article] [PubMed] [Google Scholar]
- 24.Dippel DW, Du Ry van Beest Holle M, van Kooten F, Koudstaal PJ. The validity and reliability of signs of early infarction on CT in acute ischaemic stroke. Neuroradiology 2000;42:629–633 [DOI] [PubMed] [Google Scholar]
- 25.Barber PA, Demchuk AM, Hudon ME, Pexman JH, Hill MD, Buchan AM. Hyperdense sylvian fissure MCA “dot” sign: a CT marker of acute ischemia. Stroke 2001;32:84–88 [DOI] [PubMed] [Google Scholar]
- 26.Kuroiwa T, Seida M, Tomida S, Hiratsuka H, Okeda R, Inaba Y. Discrepancies among CT, histological, and blood brain barrier findings in early cerebral ischemia. J Neurosurg 1986;65:517–524 [DOI] [PubMed] [Google Scholar]
- 27.Moulin T, Cattin F, Crepin-Leblond T, et al. Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology 1996;47:366–375 [DOI] [PubMed] [Google Scholar]
- 28.Rother J, Jonetz-Mentzel L, Fiala A, et al. Hemodynamic assessment of acute stroke using dynamic single-slice computed tomographic perfusion imaging. Arch Neurol 2000;57:1161–1166 [DOI] [PubMed] [Google Scholar]
- 29.König M, Klotz E, Luka B, Venderink DJ, Spittler JF, Heuser L. Perfusion CT of the brain: diagnostic approach for the early detection of ischemic stroke. Radiology 1998;209:85–93 [DOI] [PubMed] [Google Scholar]
- 30.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109–2118 [DOI] [PubMed] [Google Scholar]
- 31.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 32.Mohr JP, Biller J, Hilal SK, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke 1995;26:807–812 [DOI] [PubMed] [Google Scholar]
- 33.Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 1997;41:574–580 [DOI] [PubMed] [Google Scholar]
- 34.Lansberg MG, Norbash AM, Marks MP, Tong DC, Moseley ME, Albers GW. Advantages of adding diffusion-weighted magnetic resonance imaging to conventional magnetic resonance imaging for evaluation of acute stroke. Arch Neurol 2000;57:1311–1316 [DOI] [PubMed] [Google Scholar]
- 35.Lansberg MG, Albers GW, Beaulieu C, Marks MP. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 2000;54:1557–1561 [DOI] [PubMed] [Google Scholar]
- 36.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002;33:2206–2210 [DOI] [PubMed] [Google Scholar]