Abstract
BACKGROUND AND PURPOSE: Dextran-coated ultrasmall superparamagnetic iron oxide ferumoxtran-10 (Combidex) is used in reticuloendothelial MR imaging. Our purpose was to determine the optimal dose and imaging time for lymph node evaluation.
MATERIALS: Twenty-four healthy volunteers underwent neck MR imaging before and 6, 12, 24, and 36 hours after receiving 1.1, 1.7, 2.6, or 3.4 mg Fe/kg ferumoxtran-10. Vital signs, serum and urine levels, and adverse events were monitored. Qualitative nodal architecture, size, and signal-intensity changes were assessed on T1-, T2-, and T2*-weighted (fast field-echo 25° or 80° flip angle [FFE-25 or FFE-80]) images. Region-of-interest intensities were measured quantitatively.
RESULTS: Consistently strong enhancement in normal nodes was found with 24- and 36-hour T2- and T2*-weighted imaging after 2.6 and 3.4 mg Fe/kg doses. No serious adverse events occurred. With 2.6 mg Fe/kg, unblinded (vs blinded) specificities at 24 and 36 hours, respectively, were 100% and 100% (vs 88% and 88%) with T2-weighted, 96% and 96% (vs 73% and 85%) with FFE-25, and 100% and 92% (vs 85% and 88%) with FFE-80 sequences. With 3.4 mg Fe/kg, unblinded (vs blinded) specificities at 24 and 36 hours, respectively, were 89% and 79% (vs 75% and 75%) with T2-weighted, 84% and 79% (vs 95% and 100%) with FFE-25, and 95% and 79% (vs 95% and 80%) with FFE-80 sequences.
CONCLUSION: Ferumoxtran-10 nodal imaging appears to be effective and safe. Signal intensity and specificity for normal nodes were best 24 or 36 hours after 2.6 and 3.4 mg Fe/kg doses. Nodal conspicuity was best with T2- and T2*-weighted sequences.
Ferumoxtran-10 (Combidex; Advanced Magnetics Inc, Cambridge, MA) is an ultrasmall, superparamagnetic, biodegradable iron oxide particle that is covered with a low-molecular-weight dextran (1, 2). This agent accumulates in the reticuloendothelial system, taken up by macrophages in normal cervical nodes (3, 4). Preclinical studies demonstrated the potential use for ferumoxtran-10 in differentiating normal and reactive lymph nodes from metastatic lymph nodes (3, 5). Normal or reactive lymph nodes, which possess macrophages, phagocytose the contrast agent; the result is decreased signal intensity on postdose T2-weighted spin-echo and heavily T2*-weighted gradient-echo images. Metastatic lymph nodes, in which tumor replaces normal tissue and macrophages, do not take up ferumoxtran-10, and do not show any change in signal intensity on postdose images.
A phase 1 clinical trial showed that the signal intensity of normal lymph nodes changed slightly at 4 hours and significantly at 24 hours after injection of a 1.7 mg Fe/kg dose (6). The goals of this study were to determine the safety and effectiveness of four doses of ferumoxtran-10 for MR imaging of the cervical lymph nodes and to determine the appropriate time for obtaining postdose images.
Methods
Subjects
Twenty-four healthy volunteers (seven men, 17 women; age range, 18–51 years; mean age, 34 years) were admitted to our clinical research center (CRC) and underwent complete medical history and physical examination, including an assessment of their vital signs and blood and urine analyses. Inclusion criteria included healthy men and women with no history of head and neck malignancy or any other malignancy. Exclusion criteria included pregnancy or lactation; use of allergy medication three times per week; a history of autoimmune disease, asthma, reaction to contrast media, or significant drug sensitivity. No subject had a contraindication to MR imaging (eg, aneurysm clip or pacemaker). Informed written consent was obtained according to institutional review board guidelines. The subjects remained in the CRC for 2 nights and 1 day until the last MR study was performed.
History and Physical Examination
A complete medical history was obtained, and physical examination was performed within 24 hours before dose administration in all subjects who received 1.1 or 1.7 mg Fe/kg. The physical examination was repeated 24 hours after dose administration. Subjects who received 2.6 or 3.4 mg Fe/kg provided a complete medical history and underwent physical examination and 12-lead electrocardiography (ECG) within 14 days before dose administration. Physical examination was repeated at 24 and 72 hours after dose administration. ECG was repeated immediately before dose administration and at 1 and 24 hours after dose administration. In these subjects, the injection site was monitored at 10 minutes, 30 minutes, 2 hours, and 24 hours after administration for signs of irritation or inflammation.
All subjects were monitored for adverse events, defined as illness and signs or symptoms that appeared or worsened after the administration of ferumoxtran-10. If the investigator (P.A.H.) indicated that the relationship of the adverse event to therapy was definite, possible, or unknown, it was considered to be drug related. Adverse events were classified as either nonserious or serious. Nonserious events were graded as mild (resolved without treatment) or moderate (required medication or other treatment as prescribed by the investigator, but not hospitalization). Serious events were defined as those that were life threatening, were permanently disabling, required hospitalization, or resulted in death. All adverse events, whether or not they were considered to be related to the contrast agent, were recorded.
Vital Signs
Systolic and diastolic blood pressures, radial pulse rate, respiratory rate, and temperature were recorded before dose administration and at 1 and 24 hours after dose administration in subjects who received 1.1 or 1.7 mg Fe/kg. In subjects in the 2.6 or 3.4 mg Fe/kg group, vital signs were obtained within 14 days of dose administration; at baseline; and at 1, 5, 10, 15, and 30 minutes and 1, 4, 8, 24, 48, and 72 hours after dose administration.
Laboratory Tests
In all subjects, the following were assessed: 1) complete blood count, which included the red blood cell count, hemoglobin level, hematocrit level, mean corpuscular volume, total white blood cell count, differential, and platelet count; 2) serum levels of glucose, creatinine, urea nitrogen, calcium, phosphorus, uric acid, total protein, albumin, total bilirubin, alkaline phosphatase, and lactic dehydrogenase; 3) levels of electrolytes, including sodium, potassium, and chloride; 4) hepatic function, which involved gamma glutamyl transpeptidase, glutamic-oxaloacetic transaminase/aspartate aminotransferase, and glutamic-pyruvic transaminase/alanine aminotransferase levels; 5) clotting function, which included the prothrombin time and activated partial thromboplastin time; and 6) urinalysis results, which included the pH, specific gravity, protein level, glucose level, presence or absence of blood, and ketone level.
The laboratory tests were performed within 24 hours before and then 24 hours after dose administration in all subjects. Subjects in the 2.6 and 3.4 mg Fe/kg groups also underwent laboratory evaluation within 14 days before ferumoxtran-10 administration and at 8, 48, and 72 hours after dose administration, except that urinalyses were not performed at 8 hours. Subjects in the 2.6 and 3.4 mg Fe/kg groups also underwent the following additional tests: 1) baseline special screening, including a serum hepatitis B surface antigen test, rapid plasma reagin test, and urine screening for drugs of abuse; 2) ancillary tests of the total complement level, glucose-6-phosphatase dehydrogenase level, reticulocyte count, erythrocyte sedimentation rate, and bleeding time; and 3) iron metabolism panel, including tests for transferrin, serum iron, total iron binding capacity, and serum ferritin. The iron metabolism panel tests were repeated at 48 and 72 hours after dose administration.
The investigator (P.A.H.) reviewed the baseline and postdose laboratory results within 24 hours of the blood draw, determined if postdose changes were clinically important, and determined whether the changes were related to treatment.
Ferumoxtran-10
Ferumoxtran-10 (Combidex) was supplied as a lyophilized powder and reconstituted with sterile 0.9% sodium chloride solution and mixed well. When reconstituted, ferumoxtran-10 is a black-to–reddish brown aqueous colloidal solution with a superparamagnetic iron oxide concentration of 20 mg Fe/mL. The agent was then diluted to 100 mL with 0.9% saline solution and administered in a piggyback fashion through a 5-μm filter at a rate of 4 mL/min into an intravenous line. The total injection time was approximately 25 minutes.
MR Imaging
Axial images through the neck were obtained with a 1.5-T superconducting magnet by using a quadrature neck coil. Four axial sequences were performed in each subject both before and at stated intervals after the administration of contrast material. Sequences included a T1-weighted sequence (500/20/1 [TR/TE/NEX]), a fast spin-echo T2-weighted (3500/120/1) sequence, and two gradient-echo (fast field-echo [FFE]) sequences (120/7.7/1 and 25° flip angle [FFE-25] and 120/7.8/1 and 80° flip angle [FFE-80]) sequences. All were performed with 5-mm section thickness and 192 × 256 matrix. Fat-saturation techniques were not available and, therefore, not used with T2-weighted imaging.
Image Interpretation
Two reviewers evaluated the pre- and postcontrast images: 1) the study investigator (P.A.H.) a neuroradiologist who had access to all available data, including the dose, for each subject, and 2) a blinded reader (Y.A.), who was unaffiliated with the study. The blinded reader also was a neuroradiologist and did not know the doses administered or the times when the images were obtained. The images were presented to the blinded reader in random order, with no indication of the subject number, dose administered, or postdose time at which the image was obtained.
The following six cervical nodal characteristics were reviewed: First, lymph node location was determined. By using standardized anatomic diagrams, the approximate location of each visualized lymph node was marked. Each node was assigned a number, which was used in assessing the remaining characteristics.
Second, the change in signal intensity was noted. For each lymph node visualized on the predose images and for each sequence performed, the reviewer indicated whether a qualitative change in signal intensity occurred at each postdose time point. The following scale was used: 1 indicated decreased signal intensity on postdose images; 2, slightly decreased signal intensity on postdose images; 3, no change from predose images to postdose images; 4, slightly increased signal intensity on postdose images; and 5, increased signal intensity on postdose images.
Third, lymph node architecture was evaluated. For each lymph node identified and for each sequence, the reviewer indicated whether the architecture was homogeneous or heterogeneous. The degree of heterogeneity was indicated by using diagrams provided on the case report form. Nodal architecture was recorded for lymph nodes seen on predose and postdose images obtained as long as 36 hours after the dose.
Fourth, lymph node size was measured. The investigator recorded the size of each lymph node identified on T2-weighted and heavily T2*-weighted images obtained at all time points, to 36 hours. Nodal measurements determined on the images by using calipers. This measurement was used to determine if susceptibility artifacts, which could cause overestimation of nodal size, might be observed as the dose increased.
Fifth, whether additional lymph nodes were visualized after the dose was determined. The reviewers indicated whether any lymph nodes that were not detected before the dose were visualized after the dose. If so, the images that depicted the additional lymph nodes were identified, the location of the lymph nodes was indicated on diagrams provided in the report form, and the architecture and size of the lymph nodes were determined.
Sixth, the total number of lymph nodes seen before and after the dose was recorded.
Statistical Analyses
The signal intensities of the lymph nodes were measured by obtaining region-of-interest (ROI) measurements on the MR imaging workstation. ROI measurements (means and standard deviations) for three lymph nodes and adjacent muscle were recorded on the predose image in each patient, and the same nodes were measured on each postdose image at each time point.
Earlier preclinical and clinical studies, in which ferumoxtran-10 imaging results were compared with histologic results, showed that the signal intensity of normal lymph nodes decreases on postdose images as a result of uptake of the agent (3). Therefore, in the calculation of specificity based on signal intensity, lymph nodes that had a decrease or slight decrease in signal intensity were considered to represent true-negative results. Lymph nodes that had an increase, slight increase, or no change in signal intensity were considered to represent false-positive results.
Summary statistics were calculated for the percentage enhancement by using the following equations (in which SI indicates signal intensity) : Nodal enhancement = [(postdose nodal SI—predose nodal SI) ÷ predose nodal SI] × 100. Muscle enhancement = [(postdose muscle SI—predose muscle SI) ÷ predose muscle SI] × 100. Ratio of nodal enhancement to muscle enhancement = {[(postdose nodal SI/postdose muscle SI)—(predose nodal SI/predose muscle SI)] ÷ (predose nodal SI/predose muscle SI)} × 100.
A negative percentage enhancement indicated a decrease from the baseline signal intensity, and a positive enhancement indicated an increase from the baseline.
Normal lymph nodes generally have a homogeneous appearance on MR images obtained with or without ferumoxtran-10 (3). Therefore, a decrease in signal intensity in a node with homogeneous architecture is indicative of a nonmalignant lymph node. The importance of decreases in signal intensity in lymph nodes with a heterogeneous appearance is yet to be determined. Such changes could occur when normal lymph nodes take up ferumoxtran-10 at an insufficient dose or when malignant lymph nodes partially take up an adequate dose.
Two separate analyses were performed. In one analysis, based on signal intensity and lymph node architecture, lymph nodes with either homogeneous or heterogeneous architecture that had decreases or slight decreases in intensity were considered to represent true-negative results. Lymph nodes with either homogeneous or heterogeneous architecture that had increases, slight increases, or no change in intensity were considered to represent false-positive results. In the other analysis, based on signal intensity and lymph node architecture, lymph nodes with decreases or slight decreases in signal intensity were considered to represent true-negative results only if they had homogeneous architecture. All lymph nodes with heterogeneous architecture, as well as lymph nodes with homogeneous architecture that had increases, slight increase, or no change in signal intensity, were considered to represent false-positive results.
Results
All subjects underwent a precontrast MR study of the extracranial neck. The subjects then received either 1.1, 1.7, 2.6, or 3.4 mg Fe/kg doses of ferumoxtran-10. Six subjects were included in each dose group.
Each subject underwent an MR examination of the neck, with the same parameters as the precontrast study, at 6, 12, 24, and 36 hours after contrast agent administration. Two subjects from the 3.4 mg Fe/kg dose group also underwent MR imaging at 8–12 days, 1 month, and 3 months after the dose.
Safety Results
All of the adverse events that occurred were nonserious, mild, or moderate in intensity, and most were of short duration. The most commonly reported adverse events overall were hives and urticaria (five of 24 subjects), pruritus (four of 24 subjects), and headache (two of 24 subjects). One subject had a single hive that did not require treatment, two of five subjects had hives on the forearms, and two of five subjects had unspecified hives. These last four subjects were treated with diphenhydramine, without incident. No evidence suggested a relationship between the dose level and the occurrence of any specific adverse event. Most events resolved within 6 hours. Urticaria or pruritus was treated with diphenhydramine, and epigastric pain in a subject with a known hiatal hernia was treated with antacids.
No clinically important effect on the vital signs as a result of ferumoxtran-10 was observed.
Laboratory Results
Results of routine serum chemistry tests, liver function tests, hematologic tests, and urinalysis were not affected by ferumoxtran-10. Because the principal ingredient and active component of ferumoxtran-10 is iron oxide, the analyses of iron metabolism reflected the iron contributed by the agent. The amount of iron oxide is slightly more than that in a unit of blood, and changes in iron metabolism as a result of dose administration were not enough to cause iron overload in a subject with normal iron stores. At all dose levels, ferumoxtran-10 increased serum ferritin levels, serum iron levels, and the total iron binding capacity. These changes were not thought to be clinically important.
Nodal Changes
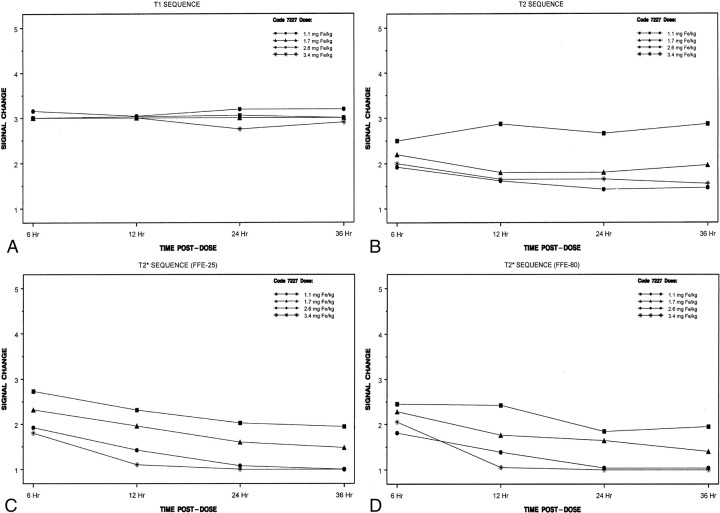
All cervical lymph nodes detected in the healthy subjects were assumed to be nonmalignant. Little quantitative change in the signal intensity over time was noted on the T1-weighted images. The results of the blinded evaluation (Fig 1) were similar to those of the unblinded evaluation. The largest decreases in signal intensity occurred with the 2.6 and 3.4 mg Fe/kg doses and the T2*-weighted sequences, and the most conspicuous loss of signal intensity occurred at 12, 24, or 36 hours after ferumoxtran-10 administration (Figs 1–3)
Fig 1.
Mean change in signal intensity over time in the blinded evaluation
A, With T1-weighted imaging.
B, With T2-weighted imaging.
C, With T2*-weighted (FFE-25) imaging.
D, With T2*-weighted (FFE-80) imaging.
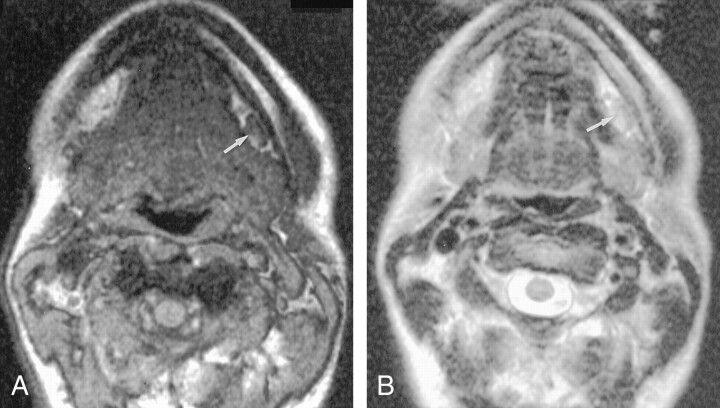
Fig 3.
Axial MR images obtained 36 hours after the intravenous administration of 2.6 mg. Fe/kg ferumoxtran-10 in the same volunteer as in Figure 2.
A, Gradient-echo (FFE-80) (120/7.8/1, 80° flip angle) image shows that the benign node is now homogeneously hypointense (white arrow). Note the multiple additional nodes, some of which are delineated by black arrows, all of which are better visualized now than before contrast enhancement.
B,T2-weighted (3500/120/1) image obtained at the same level as Figure 1B shows excellent contrast between the enhanced left submandibular node (arrow) and the subcutaneous fat.
Nodes with increases or no change in signal intensity were considered to represent false-positive results. Heterogeneous architecture was also considered to represent a false-positive result, because normal nodes should homogeneously enhance with ferumoxtran-10. The specificity, from both the unblinded and blinded evaluations, were high for T2- and T2*-weighted sequences when images were obtained 24 and 36 hours after the 2.6 mg Fe/kg dose (Tables 1 and 2). At a dose of 2.6 mg Fe/kg, the unblinded evaluation had a specificity of 100% at 24 and 36 hours with the T2-weighted sequence, 96% at 24 and 36 hours with the FFE-25 sequence, and 100% at 24 hours and 92% at 36 hours for the FFE-80 sequence. The blinded specificities were slightly lower for the same dose and were 88% at 24 and 36 hours with T2-weighted images, 73% at 24 hours and 85% at 36 hours with FFE-25 images, and 85% at 24 hours and 88% at 36 hours with FFE-80 images.
TABLE 1:
Specificity in the unblinded evaluation
| Dose Group and Postdose Time Point | Total Lymph Nodes | Sequence Specificity (%) |
||
|---|---|---|---|---|
| T2-Weighted | FFE-25 | FFE-80 | ||
| 1.1 mg Fe/kg | ||||
| 6 h | 41 | 78 | 12 | 20 |
| 12 h | 41 | 90 | 56* | 74* |
| 24 h | 41 | 80 | 62* | 87* |
| 36 h | 41 | 56 | 62* | 65† |
| 1.7 mg Fe/kg | ||||
| 6 h | 25 | 88 | 32 | 44 |
| 12 h | 25 | 92 | 64 | 84 |
| 24 h | 25 | 100 | 72 | 84 |
| 36 h | 25 | 92 | 84 | 76 |
| 2.6 mg Fe/kg | ||||
| 6 h | 25 | 92 | 56 | 68 |
| 12 h | 25 | 96 | 84 | 96 |
| 24 h | 25 | 100 | 96 | 100 |
| 36 h | 25 | 100 | 96 | 92 |
| 3.4 mg Fe/kg | ||||
| 6 h | 19 | 95 | 63 | 89 |
| 12 h | 19 | 100 | 83‡ | 95 |
| 24 h | 19 | 89 | 84 | 95 |
| 36 h | 19 | 79 | 79 | 79 |
Note.—The specificity was based on the signal intensity and lymph node architecture. A decrease in the signal intensity of a heterogeneous lymph node indicated a false-positive result.
The total number of lymph nodes was 39.
The total number of lymph nodes was 40.
The total number of lymph nodes was 18.
TABLE 2:
Specificity in the blinded evaluation
| Dose Group and Postdose Time Point | Total Lymph Nodes | Sequence Specificity (%) |
||
|---|---|---|---|---|
| T2-Weighted | FFE-25 | FFE-80 | ||
| 1.1 mg Fe/kg | ||||
| 6 h | 38 | 45 | 16* | 37 |
| 12 h | 38 | 11 | 40† | 47 |
| 24 h | 38 | 29 | 53 | 66 |
| 36 h | 38 | 14* | 50 | 50 |
| 1.7 mg Fe/kg | ||||
| 6 h | 25 | 60 | 52 | 52 |
| 12 h | 25 | 80 | 32 | 68 |
| 24 h | 25 | 88 | 48 | 64 |
| 36 h | 25 | 80 | 48 | 64 |
| 2.6 mg Fe/kg | ||||
| 6 h | 26 | 88 | 42 | 50 |
| 12 h | 26 | 81 | 58 | 54 |
| 24 h | 26 | 88 | 73 | 85 |
| 36 h | 26 | 88 | 85 | 88 |
| 3.4 mg Fe/kg | ||||
| 6 h | 20 | 60 | 55 | 35 |
| 12 h | 20 | 70 | 70 | 80 |
| 24 h | 20 | 75 | 95 | 95 |
| 36 h | 20 | 75 | 100 | 80 |
Note.— The specificity was based on the signal intensity and lymph node architecture. A decrease in the signal intensity of a heterogeneous lymph node indicated a false-positive result.
The total number of lymph nodes was 37.
The total number of lymph nodes was 35.
At a dose of 3.4 mg Fe/kg, specificities for the unblinded reader were 89%, 84% and 95% at 24 hours with T2-weighted, FFE-25, and FFE-80 sequences, respectively; the specificity was 79% for all three sequences at 36 hours. The blinded evaluation had specificities of 75%, 95%, and 95% at 24 hours for T2-weighted, FFE-25, and FFE-80 sequences, respectively, and 75%, 100%, and 80% at 36 hours, respectively.
No lymph nodes that had not been present on the predose images were detected on the postdose images. No gross changes were noted on the postdose images, compared with the predose images, with the three lower doses. Specifically, no size or contour changes occurred between the pre and postdose images, as the readers noted. However, in the 3.4 mg Fe/kg group, gross changes were visible on the postdose images in five of the six subjects. The borders of the nodes and discrete nodes in a cluster of nodes were better seen with the highest dose on the 36-hour postdose scan.
Overall, lymph node size changed only minimally (mean, ≤0.3 mm) from before the dose to after the dose.
Signal intensity measurements were recorded in three lymph nodes in each subject. The signal intensity of lymph nodes decreased on T2- and T2*-weighted images obtained after the administration of all four doses of ferumoxtran-10; the largest decreases occurred with doses of 2.6 and 3.4 mg Fe/kg (Table 3). For the T2-weighted sequences, the signal intensity decreased over time, to 24 hours, and then appeared to return toward baseline at 36 hours. With the T2- and T2*-weighted sequences, when values were recorded beyond 36 hours (only with the highest dose), signal intensity returned to baseline levels at 10 days and 3 months, respectively. Small decreases in the signal intensity of muscle were noted at most imaging times with the T2- and T2*-weighted sequences, although occasional increases were noted. The decreases were not as large or as consistent as those seen with lymph nodes.
TABLE 3:
Descriptive statistics for quantitative signal intensity measurements of lymph nodes
| Dose Group and Time Point | Total Lymph Nodes | Quantitative Signal Intensity Measurements by Sequence* |
|||
|---|---|---|---|---|---|
| T1-Weighted | T2-Weighted | FFE-25 | FFE-80 | ||
| 1.1 mg Fe/kg | |||||
| Predose | 18 | 486.1 + 45.8 | 536.0 + 64.6 | 1170.6 + 179.3 | 784.9 + 98.8 |
| 6 h | 18 | 626.6 + 70.3 | 277.1 + 45.4 | 995.6 + 129.4 | 510.6 + 69.7 |
| 12 h | 18 | 587.9 + 53.7 | 297.3 + 55.1 | 906.4 + 197.1 | 524.3 + 143.6 |
| 24 h | 18 | 596.9 + 98.2 | 280.1 + 34.7 | 904.7 + 178.9 | 442.4 + 117.7 |
| 36 h | 18 | 570.3 + 92.2 | 323.0 + 40.5 | 852.9 + 231.4 | 447.3 + 137.9 |
| 1.7 mg Fe/kg | |||||
| Predose | 18 | 498.5 + 59.0 | 505.5 + 48.6 | 979.7 + 283.4 | 669.5 + 139.8 |
| 6 h | 15 | 618.7 + 89.1 | 256.1 + 67.0 | 901.3 + 257.9 | 473.8 + 167.6 |
| 12 h | 18 | 555.2 + 81.3 | 275.8 + 35.4 | 725.7 + 276.5 | 372.5 + 150.3 |
| 24 h | 18 | 567.9 + 101.0 | 279.9 + 74.6 | 676.7 + 266.4 | 341.7 + 135.5 |
| 36 h | 18 | 545.0 + 93.0 | 291.5 + 46.6 | 491.3 + 171.6 | 311.7 + 137.6 |
| 2.6 mg Fe/kg | |||||
| Predose | 18 | 470.3 + 55.4 | 561.2 + 71.6 | 1150.0 + 125.2 | 777.1 + 95.2 |
| 6 h | 18 | 568.1 + 80.0 | 293.8 + 54.5 | 754.2 + 148.1 | 493.3 + 116.5 |
| 12 h | 18 | 534.7 + 109.7 | 225.7 + 50.4 | 535.6 + 93.6 | 328.1 + 64.1 |
| 24 h | 18 | 511.3 + 85.4 | 196.6 + 42.7 | 335.2 + 85.9 | 243.2 + 66.5 |
| 36 h | 18 | 494.6 + 119.3 | 209.2 + 41.9 | 316.3 + 79.4 | 188.4 + 48.6 |
| 3.4 mg Fe/kg | |||||
| Predose | 18 | 515.0 + 76.9 | 583.6 + 140.7† | 1132.2 + 164.7 | 827.2 + 129.3 |
| 6 h | 18 | 608.3 + 68.8 | 276.8 + 110.9† | 710.8 + 166.4 | 550.6 + 117.9 |
| 12 h | 18 | 559.9 + 74.9 | 219.8 + 63.9† | 452.5 + 125.3 | 346.5 + 72.2 |
| 24 h | 18 | 513.1 + 68.6 | 178.3 + 58.7† | 326.2 + 98.4 | 255.4 + 76.9 |
| 36 h | 18 | 459.4 + 108.4 | 206.8 + 69.8† | 300.6 + 72.6 | 221.9 + 35.5 |
| 10 d | 6 | 487.7 + 58.2 | 570.9 + 113.2‡ | 594.1 + 80.6 | 530.8 + 103.8 |
| 1 mo | 6 | 457.8 + 81.2 | 561.3 + 115.4‡ | 726.9 + 450.8 | 617.3 + 185.4 |
| 3 mo | 6 | 502.6 + 14.0 | 595.3 + 27.7‡ | 965.2 + 272.3 | 895.8 + 181.5 |
Data are the mean + the SD.
The total number of lymph nodes was 17.
The total number of lymph nodes was 5.
Discussion
The presence of nodal metastatic disease in a patient with squamous cell carcinoma of the head and neck is one of the most important prognostic factors (7). The size criteria at CT or MR imaging that are currently used at our institution include a short axial nodal diameter and level I and II nodes larger than 11 mm and larger than 10 mm in other nodal chains; these findings are considered positive in a patient with squamous cell carcinoma of the head and neck (8). Imaging criteria, however, are imprecise; their sensitivity in the detection of nodal metastatic disease is reported to be as low as 60% (9). In one large series, 67% of malignant nodes were 10.0 mm or smaller (10). Lymph node metastases from colon cancer may be smaller than 5 mm in diameter in 65% of the cases (11). Nodal necrosis, even in a normal-sized node, is accepted as a reliable predictor of metastatic disease. Furthermore, heterogeneity of nodal attenuation or signal intensity likely represents small tumor nodules. Unfortunately, microscopic spread of disease, which cannot be detected with size criteria or a heterogeneous appearance on MR or CT images, may be present. Nodes that are homogeneous, non-necrotic, and not enlarged may harbor microscopic metastatic disease, but they cannot be detected with current imaging methods, most of which rely on anatomic characteristics in the evaluation of nodal disease.
The sensitivity of MR imaging in detecting metastatic cervical adenopathy appears to be no better than that of CT when size and necrosis criteria are used (12). Even nodal signal intensity on MR images cannot be used to differentiate metastatic nonnecrotic nodes from reactive adenopathy (13–16). Clearly, although the morphologic appearance (size and necrosis) of a lymph node on CT or MR images is more sensitive than physical examination findings alone, the anatomic nodal characteristics fall far short of the acceptable measures of sensitivity and specificity.
Ferumoxtran-10 is a low-molecular-weight dextran-coated iron oxide colloid with a long plasma half-life. It has a long blood half-life in humans (25–30 hours), and it is eventually phagocytosed by macrophages in the liver, spleen, bone marrow, and lymph nodes. In the late phase of its distribution, it can be used to image lymph nodes. Although the delayed imaging after contrast agent administration may be a minor inconvenience for the patient, it is no different from the delayed imaging often required in routine nuclear medicine studies. The advantages, especially the ability to detect metastatic nodal disease in normal-sized nodes (17), far outweighs the minor disadvantage of the patient’s having to return to the MR suite for imaging 24–36 hours after drug administration. Preclinical studies have demonstrated the potential use of ferumoxtran-10–enhanced MR imaging to differentiate normal and reactive lymph nodes from metastatic lymph nodes (5, 17).
For both the unblinded and blinded evaluations, the highest specificity rates (73–100%) were achieved with doses of 2.6 or 3.4 mg Fe/kg when images were obtained either 24 or 36 hours after injection of ferumoxtran-10. Lower specificity rates at doses of 1.1 and 1.7 mg Fe/kg were due to an increase, no change, or only a mild decrease in signal intensity on the T2-weighted and gradient-echo images.
Normal nodes may have small areas that lack a signal void after ferumoxtran-10 administration. Asymmetric contrast agent uptake within nodes occasionally occurs, and this effect may simulate tumoral involvement in the node. The distribution of iron oxide particles within a node is not homogeneous. The contrast material concentrates in the marginal sinuses, or medullary sinusoid, but it spares the lymphoid follicles. Macrophages are located in the marginal sinus, have phagocytic capability, and concentrate the contrast; the result is a region of signal void. Lymphoid follicles are filled with lymphocytes that do not have a phagocytic function. This feature has been shown with microscopic MR imaging, and it is potentially a cause of false-positive results (18).
Theoretically, potential causes of false-negative results could be present, especially on gradient-echo images. In fact, because of the blooming effect, nodes may appear slightly larger after ferumoxtran-10 administration. This blooming effect could potentially obscure a small focus of tumor. Susceptibility artifact is less severe on T2-weighted images and, ultimately, they may be the single best postcontrast sequence, to decrease the potential for obscuring small tumor foci. Data from a large series of metastatic nodal disease will likely help in determining whether heterogeneous enhancement and blooming artifact is clinically relevant.
In summary, based on qualitative results of the blinded evaluation, the specificity of ferumoxtran-10 in the detection of normal lymph nodes was consistently high with T2-weighted and T2*-weighted images obtained 24 and 36 hours after the administration of 2.6 and 3.4 mg Fe/kg doses. The specificities for the two gradient-echo sequences did not differ, and no obvious advantage of one over the other was noted. The qualitative results of the unblinded evaluation were similar to those of the blinded evaluation. The specificities were highest with the 2.6 mg Fe/kg dose when images were obtained 24 and 36 hours after dose administration. Signal intensity data and specificity rates in the detection of normal lymph nodes showed that the best imaging results were achieved with doses of 2.6 and 3.4 mg Fe/kg when the MR images were obtained 24 or 36 hours after ferumoxtran-10 administration. No substantial differences between the two doses or the two time points was noted. The most substantial change in signal intensity occurred with T2- and T2*-weighted sequences; no noticeable change in signal intensity was reported with any dose at any time point with the T1-weighted sequences. Finally, changes in lymph node size from before the dose to after the dose with any sequence or dose were negligible.
Conclusion
Consistently high specificity, or decreased enhancement of normal nodes, for the T2-weighted and T2*-weighted sequences occurred with doses of 2.6 and 3.4 mg Fe/kg when the images were obtained 24 and 36 hours after dose administration. At a dose of 2.6 mg Fe/kg, the unblinded evaluation had a specificity of 100% at 24 and 36 hours with the T2-weighted sequence, 96% at both times with the FFE-25 sequence, and 100% at 24 hours and 92% at 36 hours with the FFE-80 sequence. The blinded specificities were slightly lower for the same dose and were 88% at 24 and 36 hours with T2-weighted images, 73% at 24 hours and 85% at 36 hours with FFE-25 images, and 85% at 24 hours and 88% at 36 hours with FFE-80 images. At a dose of 3.4 mg Fe/kg, specificities for the unblinded reader were 89%, 84% and 95% at 24 hours for T2-weighted, FFE-25, and FFE-80 imaging, respectively; the specificity at 36 hours was 79% for all three sequences. The blinded evaluation had specificities of 75%, 95%, and 95% at 24 hours for T2-weighted, FFE-25, and FFE-80 imaging, respectively, and specificities were 75%, 100%, and 80% at 36 hours, respectively.
At these time points and doses, normal nodes had the most marked decreases in signal intensity after ferumoxtran-10 administration. This agent is safe; no severe adverse events occurred in this series. The results of this open-label, single-center, dose-ranging, phase 2 study will help in determining the optimal dose and postdose timing of imaging in patients with suspected metastatic nodal disease.
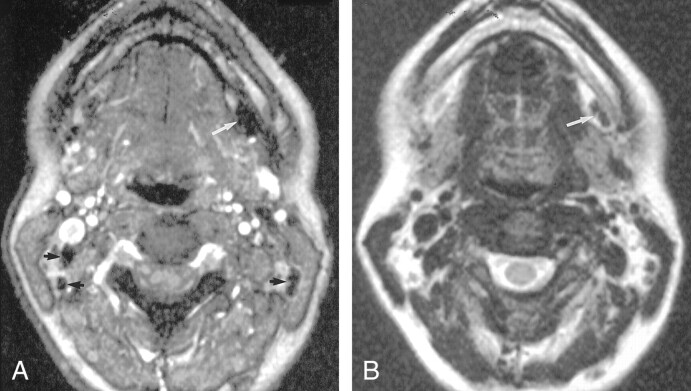
Fig 2.
Axial nonenhanced MR images in a healthy volunteer
A, Gradient-echo (FFE-80) (120/7.8/1, 80° flip angle) image shows a small left submandibular node (arrow).
B, On the fast spin-echo T2-weighted (3500/120/1) image, the node (arrow) is difficult to visualize because it is nearly isointense to fat.
Footnotes
Supported in part by a grant from Advanced Magnetics, Inc.
Presented at the 34th Annual Meeting, American Society of Neuroradiology, June 1996, Seattle, WA.
References
- 1.Jung CW. Surface properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging 1995;13 ;675–691 [DOI] [PubMed] [Google Scholar]
- 2.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging 1995;13:661–674 [DOI] [PubMed] [Google Scholar]
- 3.Anzai Y, Prince MR. Iron oxide-enhanced MR lymphography: The evaluation of cervical lymph node metastases in head and neck cancer. J Magn Reson Imaging 1997;7:75–81 [DOI] [PubMed] [Google Scholar]
- 4.Anzai Y, McLachlan S, Morris M, et al. Dextran-coated superparamagnetic iron oxide: An MR contrast agent for assessing lymph nodes in the head and neck. AJNR Am J Neuroradiol 1994;15:87–94 [PMC free article] [PubMed] [Google Scholar]
- 5.Vassallo P, Matei C, Heston WDW, et al. AMI-227-enhanced MR lymphography: Usefulness for differentiating reactive from tumor-bearing lymph nodes. Radiology 1994;193:501–506 [DOI] [PubMed] [Google Scholar]
- 6.McLachlan SJ, Morris MR, Lucas MA, et al. Phase I clinical evaluation of a new iron oxide MR contrast agent. J Magn Reson Imaging 1994;301–307 [DOI] [PubMed]
- 7.Batsakis JG. Tumors of the head and neck: clinical and pathological considerations. 2nd ed. Baltimore, Md: Williams & Wilkins;1979. :240–251
- 8.Van den Brekel MWM, Stel HV, Castelijns JA, et al. Cervical lymph node metastases: assessment of radiological criteria. Radiology 1990;177:379–384 [DOI] [PubMed] [Google Scholar]
- 9.Close JG, Merkel M, Vuitch MF, et al. Computed tomographic evaluation of regional lymph node involvement in cancer of the oral cavity and oropharynx. Head Neck 1989;11:309–317 [DOI] [PubMed] [Google Scholar]
- 10.Don DM, Anzai Y, Lufkin RB, et al. Evaluation of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Laryngoscope 1995;105:669–674 [DOI] [PubMed] [Google Scholar]
- 11.Herrera-Ornales L, Justiniano J, Castillo N, et al. Metastases in small lymph nodes from colon cancer. Arch Surg 1987;122:1253–1256 [DOI] [PubMed] [Google Scholar]
- 12.Yousem D, Som PM, Hackney DB, et al. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology 1992;182:753–759 [DOI] [PubMed] [Google Scholar]
- 13.Dooms GC, Hricak H, Crooks LE, et al. Magnetic resonance imaging of the lymph nodes: comparison with CT. Radiology 1984;153:719–728 [DOI] [PubMed] [Google Scholar]
- 14.Dooms GC, Hricak H, Moseley MR, et al. Characterization of lymphadenopathy by magnetic relaxation times: preliminary results. Radiology 1985;155:691–697 [DOI] [PubMed] [Google Scholar]
- 15.Glazer G, Orringer M, Chenevert T, et al. Mediastinal lymph nodes: relaxation time/pathologic correlation and implications in staging of lung cancer with MR imaging. Radiology 1988;168:429–431 [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Heiken J, Ling D, et al. Magnetic resonance imaging of abdominal and pelvic lymphadenopathy. Radiology 1984;153:181–188 [DOI] [PubMed] [Google Scholar]
- 17.Anzai Y, Blackwell KE, Hirschowitz SL, et al. Initial clinical experience with dextran-coated superparamagnetic iron oxide for detection of lymph node metastases in patients with head and neck cancer. Radiology 1994;192:709–715 [DOI] [PubMed] [Google Scholar]
- 18.Lee AS, Weissleder R, Brady TJ, et al. Lymph nodes: microstructural anatomy at MR imaging. Radiology 1991;178:519–522 [DOI] [PubMed] [Google Scholar]