Abstract
BACKGROUND AND PURPOSE: Methotrexate is a major cause of treatment-related acute neurotoxicity in children with hematologic malignancies. The purpose of this study was to investigate whether diffusion-weighted MR imaging (DWI) detects acute methotrexate white matter neurotoxicity in this patient population.
METHODS: Six children—three female and three male—with hematologic malignancies were studied at time of onset of neurologic dysfunction during the delayed intensification or consolidation phase of therapy, when intensive intrathecal methotrexate is given. MR imaging including DWI was performed on 1.5 T MR scanners.
RESULTS: DWI demonstrated abnormal restriction of motion of water in the centrum semiovale in all six patients. This finding correlated to the acute onset of hemiparesis or aphasia. Fluid-attenuated inversion recovery imaging was not positive at this time, but it was positive in all five patients in whom follow-up imaging was performed.
CONCLUSION: Early detection of methotrexate white matter injury by DWI has the potential to alert the oncologist to this event and provide a technique by which treatment of neurotoxicity can be monitored.
Central nervous system (CNS) complications of leukemia and lymphoma treatment have been well documented. Acute neurotoxicity includes seizures, transient ischemic attacks, encephalopathy, ataxia, and myelopathy. The incidence of acute CNS neurotoxicity associated with therapy for acute lymphoblastic leukemia (ALL) is 5%–18% (1–4). Offending agents include methotrexate, cytarabine, vincristine, asparaginase, and corticosteroids.
Methotrexate is often implicated as the major cause of acute neurotoxicity. Risk factors for methotrexate-induced neurotoxicity include high-dose treatment (5), intrathecal treatment (6), young age (4, 7), and association with cranial radiation (7). Methotrexate toxicity is often associated with damage to the CNS white matter, termed “leukoencephalopathy” (LEP). On MR imaging, the hallmark of LEP is hyperintensities on T2-weighted imaging. In methotrexate-induced neurotoxicity, these T2 hyperintensities are typically located in the periventricular white matter, particularly in the centrum semiovale (7–9). For patients with ALL, the incidence of LEP associated with methotrexate treatment is between 9% and 53% (2). The frequency of LEP in a sample of ALL patients with symptoms of acute neurotoxicity is 67% and exceeds 75% for those treated with regimens that include intravenous—as opposed to oral—methotrexate (2). Despite white-matter changes on MR imaging, patients often recover spontaneously from methotrexate-induced neurotoxic events, including encephalopathy and strokelike events (10).
Because standard treatment for hematopoietic malignancies includes multimodality therapy, isolating the offending agent during an acute neurotoxic event can be difficult. Although standard MR imaging may be useful (LEP with methotrexate or sagittal sinus thrombosis with l-asparaginase), often no abnormalities are found at the time of MR imaging (1, 2). Diffusion-weighted MR imaging (DWI) has become important in imaging CNS abnormalities such as infarction, infection, or tumor and is a sensitive way of detecting cytotoxic edema. In this article, we report DWI abnormalities in patients with subacute neurotoxicity during treatment for hematologic malignancies. We believe these MR imaging changes represent an early manifestation of toxicity secondary to methotrexate.
Methods
DWI became part of the standard brain MR imaging protocol for all patients at the Children’s Hospital of Philadelphia in June 1998. To assess its utility in evaluating patients with subacute neurotoxicity during chemotherapy, we performed a retrospective review of patients with hematopoietic malignancy from June 1998 through August 2002. The cases were collected by searching the Children’s Hospital of Philadelphia Division of Oncology data base for patients who had CNS neurotoxicity as well as conducting a survey of clinicians in the Division of Oncology. We identified 35 patients with clinical evidence of neurotoxicity. There were 27 cases of ALL, 3 cases of acute myeloid leukemia (AML), 2 cases of Burkitt lymphoma, 2 cases of lymphoblastic lymphoma, and 1 case of large-cell lymphoma.
Symptoms included headaches, seizures, paresthesias, aphasia, hemiparesis, or similar symptoms. The medical records were reviewed with attention to treatment protocol, time of onset of the neurologic events, relationship to intrathecal methotrexate dose, recovery from the event, and neuroimaging.
In 28 of the 35 cases MR images were obtained with 1.5T scanners (Siemens SP, Erlangen, Germany). Five patients were not imaged at the time of the neurologic event or immediately thereafter. Two patients underwent CT only. In these two cases, the CT showed some volume loss but not acute abnormality.
MR imaging was performed for the remaining 28 patients following the neurologic event. One of the 28 patients, a 22-month-old boy with AML with 7th nerve palsy, had hemorrhage within the brain and the right globe. A second patient, a 37-month-old girl with ALL, presented with bilateral miosis. MR imaging revealed right iris infiltration. In 13 cases, MR imaging findings, including DWI, were either normal or showed brain volume loss. Of the remaining 13 patients, 7 had neurologic events associated with elevated blood pressure and on MR imaging had fluid-attenuated inversion recovery (FLAIR) abnormality and associated increased diffusion of the cortex and subcortical white matter consistent with posterior reversible encephalopathy secondary to hypertension (11, 12) and therefore were not considered for further investigation.
The remaining six patients had neurologic events whose clinical presentation and course were consistent with possible methotrexate toxicity. In addition, there was no other obvious etiology for the neurologic event (eg, tumor, hemorrhage, hypertension). MR imaging revealed DWI abnormalities, which are reported in this article. The imaging sequences consisted of sagittal spin-echo T1, axial turbo spin-echo (TSE) T2, FLAIR, and DWI, coronal TSE T2 and FLAIR, and T1 imaging in three planes following intravenous injection of gadopentetate dimeglumine (0.1 mmol/Kg).
This study received approval by the Children’s Hospital of Philadelphia institutional review board.
Results
The clinical characteristics of the six patients and description of neurotoxic events are listed in Tables 1 and 2. Mean age at time of event was 12.9 years (range, 6.75–16.5 years). There were three boys and three girls. Four of the patients had ALL and two had non-Hodgkin lymphoma. All patients received multiagent chemotherapy, which included vincristine, corticosteroids, cytarabine, and intrathecal methotrexate. Five of the patients received asparaginase as well. No patients had received cranial irradiation before the event. Four of six patients were in the delayed intensification phase of their chemotherapy protocol. All events occurred between 6 and 11 days after intrathecal methotrexate administration. Five patients had evidence of hemiparesis. Two patients had aphasia. All but one patient had resolution of symptoms within 1 week. The other had symptom resolution within 1 month. Four of the patients received no further intrathecal methotrexate. No patient had a recurrence of symptoms.
TABLE 1:
Clinical characteristics of patients
| Patient | Sex | Diagnosis | Age at Diagnosis (years) | Protocol and Phase of Treatment When Event Occurred |
|---|---|---|---|---|
| 1 | F | HR-ALL | 16.3 | CCG-1961 consolidation |
| 2 | F | Lymphoblastic lymphoma | 14.8 | CCG-A5791-like DI no. 1/1 |
| 3 | M | SR-ALL | 6.8 | CCG-1991 DI no. 1/2 |
| 4 | M | HR-ALL | 16.4 | CCG-1961 DI no. 2/2 |
| 5 | F | HR-ALL | 12.7 | CCG-1961 DI no. 2/2 |
| 6 | M | Large cell lymphoma | 9.7 | CCG-5961 |
Note.—F indicates female; M, male; ALL, acute lymphoblastic leukemia; HR, high-risk; SR, standard-risk; CCG, Children’s Cancer Group; DI, delayed intensification.
TABLE 2:
Characteristics of neurotoxic events and MRI findings
| Patient | Time between Diagnosis and Event (months) | Event | Time between IT MTX and Event (days) | Time between Event and Initial MRI (days) | 1st MRI DWI Findings | Time between Initial and Follow-up MRI (days) | Follow-up MRI FLAIR Findings | Duration of Symptoms |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | R hemiparesis, headache, dysmetria | 8 | 0 | Restricted L > R | 5 | Increased | 1 mo |
| 2 | 8 | Aphasia, headache, R hemiparesis | 7 | 1 | Restricted L > R | ND | ND | 4 d |
| 3 | 5 | L hemiparesis | 11 | 1 | Restricted R > L | 59 | Increased | 7 d |
| 4 | 11 | R hemiparesis | 6 | 1 | Restricted L only | 4 | Increased | 7 d |
| 5 | 11 | R hemiparesis | 7 | 2 | Restricted L > R | 20 | Increased | 7 d |
| 6 | 3 | Aphasia | 11 | 1 | Restricted R = L | 23 | Increased | 1 d |
Note.—R indicates right; L, left; IT MTX, intrathecal methotrexate; DWI, diffusion-weighted imaging; ND, not done; FLAIR, fluid attenuated inversion recovery sequence.
In all six cases, the initial MR imaging scan showed abnormal restricted diffusion in the centrum semiovale and no abnormality on the FLAIR or T2-weighted sequence (Table 2). In four of six initial studies, the abnormal diffusion was either limited to or more prominent in the left centrum semiovale. In addition, if there was a focal neurologic event, it was congruent with the site of the DWI findings (Table 2). Five of the six patients had follow-up MR imaging, and in all cases there was resolution of the diffusion abnormality and interval development of abnormal signal intensity on FLAIR imaging and T2WI. FLAIR imaging and T2WI were positive as early as 4 days after initial MR imaging.
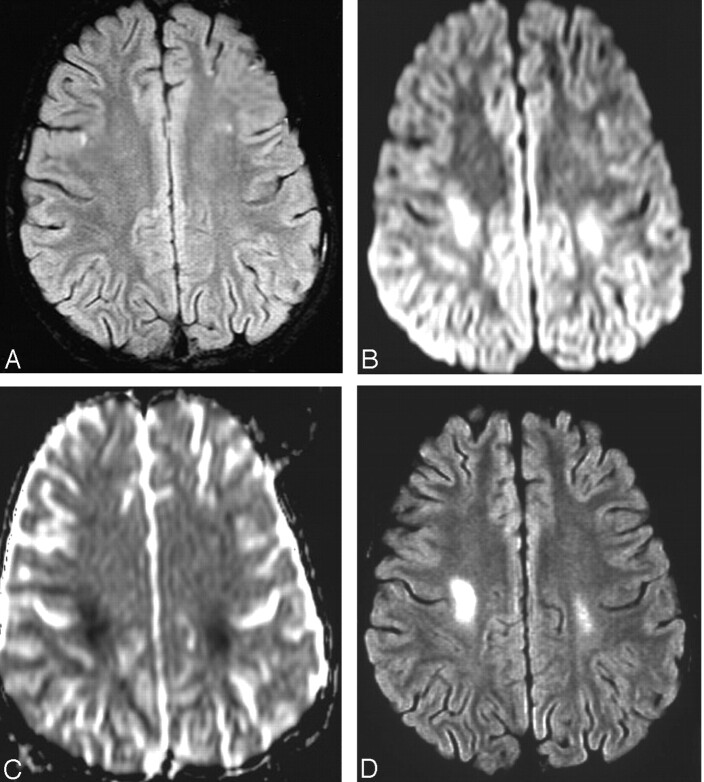
Figure 1 shows a representative case (case 3). The patient presented 11 days following intrathecal methotrexate with gait disturbance and left hemiparesis. Figures 1B and 1C show the abnormality of diffusion in the centrum semiovale bilaterally. FLAIR was not positive (Fig 1A) at the time of initial imaging. On follow-up MR imaging 2 months later, FLAIR imaging was positive (Fig 1D) in the same location as the DWI had been.
Fig 1.
Patient 3. MR imaging findings at the time of neurologic event (A–C) and at follow-up 2 months later (D).
A, Axial fat-suppressed FLAIR image (TR = 9000 msec; TE = 112 msec; TI = 2500 msec; acq = 1) shows no abnormality.
B, Axial diffusion image (B = 1000 s/mm2) shows high signal intensity at site of restricted motion of water in the parietal white matter of both hemispheres.
C, Axial ADC map shows hypointensity at affected sites. ADC measurements 50 s/mm2 (normal = 95 s/mm2).
D, Axial fat suppressed FLAIR image (TR = 9000 msec; TE = 112 msec; 1 = 2500 msec; acq = 1) shows bilateral abnormally increased signal intensity in white matter, right greater than left.
Discussion
We report six children who had subacute neurotoxicity during treatment for hematologic malignancies. Although these children were receiving other agents (cytarabine, corticosteroids, vincristine, and asparaginase) that cause neurotoxicity, the description of events, timing in relation to intrathecal methotrexate administration, duration of symptoms, and subsequent abnormalities on FLAIR imaging are consistent with subacute toxicity secondary to methotrexate (3, 10, 13, 14).
The pathophysiology of methotrexate neurotoxicity is unclear. Several mechanisms have been proposed. These include increased adenosine accumulation (15), homocysteine elevation and its excitatory effects on the n-methyl-d-aspartate (NMDA) receptor (16), and alterations of biopterin metabolism (2). The MR imaging findings of increased signal intensity on DWI with hypointensity on apparent diffusion coefficient (ADC) map are indicative of cytotoxic edema (17). This is consistent with the proposed mechanisms of a direct neurotoxic effect of methotrexate on the cell. Although lesions with low ADC values are usually associated with permanent injury (17), the resolution of symptoms in our patients suggests that this acute, methotrexate-induced cellular swelling is not necessarily irreversible.
Treatment attempts for methotrexate neurotoxicity have included aminophylline (15, 18), an adenosine antagonist, and dextromethorphan (16), an antagonist of the NMDA receptor. Some anecdotal success has been reported, but no large series have confirmed the efficacy of either agent.
In our report, DWI in all cases revealed abnormalities in the deep periventricular white matter that preceded abnormalities in the same region by FLAIR imaging. Because patients are receiving multiple chemotherapy agents, the etiology of a neurotoxic event can be difficult to define, particularly when standard MR imaging is negative. The finding of deep white matter changes on DWI at the time of event may implicate methotrexate as the etiologic agent and influence decision making regarding whether treatment with methotrexate should be continued. Although no standard therapy for methotrexate neurotoxicity currently exists, earlier diagnosis of an event with DWI would potentially allow for earlier intervention with new therapies as they arise. It might also be used as part of eligibility criteria for testing new agents for treatment of methotrexate neurotoxicity. Future prospective studies of DWI in a larger cohort of children with neurotoxicity should be undertaken to confirm these findings.
Conclusion
Early detection of methotrexate white matter injury by DWI has the potential to alert the oncologist to this event and provide a technique by which neurotoxicity can be monitored.
Footnotes
Presented at the 41st annual meeting of the American Society of Neuroradiology, Washington, DC, April 28–May 2, 2003.
References
- 1.Lo Nigro L, Di Cataldo A, Schiliro G. Acute neurotoxicity in children with B-lineage acute lymphoblastic leukemia (B-ALL) treated with intermediate risk protocols. Med Pediatr Oncol 2000;35:449–455 [DOI] [PubMed] [Google Scholar]
- 2.Mahoney DH Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy: a Pediatric Oncology Group study. J Clin Oncol 1998;16:1712–1722 [DOI] [PubMed] [Google Scholar]
- 3.Shuper A, Stark B, Kornreich L, et al. Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. J Child Neurol 2000;15:573–580 [DOI] [PubMed] [Google Scholar]
- 4.Chessells JM, Cox TC, Kendall B, et al. Neurotoxicity in lymphoblastic leukaemia: comparison of oral and intramuscular methotrexate and two doses of radiation. Arch Dis Child 1990;65:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe N, Takaue Y, Anzai T, Robertson R. Transient neurologic disturbances induced by high-dose methotrexate treatment. Cancer 1985;56:1356–1360 [DOI] [PubMed] [Google Scholar]
- 6.Gowan GM, Herrington JD, Simonetta AB. Methotrexate-induced toxic leukoencephalopathy. Pharmacotherapy 2002;22:1183–1187 [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Takahashi S, Sato A, et al. Leukoencephalopathy in childhood hematopoietic neoplasm caused by moderate-dose methotrexate and prophylactic cranial radiotherapy: an MR analysis. Int J Radiat Oncol Biol Phys 1995;32:913–918 [DOI] [PubMed] [Google Scholar]
- 8.Lien HH, Blomlie V, Saeter G, et al. Osteogenic sarcoma: MR signal abnormalities of the brain in asymptomatic patients treated with high-dose methotrexate. Radiology 1991;179:547–550 [DOI] [PubMed] [Google Scholar]
- 9.Asato R, Akiyama Y, Ito M, et al. Nuclear magnetic resonance abnormalities of the cerebral white matter in children with acute lymphoblastic leukemia and malignant lymphoma during and after central nervous system prophylactic treatment with intrathecal methotrexate. Cancer 1992;70:1997–2004 [DOI] [PubMed] [Google Scholar]
- 10.Keime-Guibert F, Napolitano M, Delattre JY. Neurological complications of radiotherapy and chemotherapy. J Neurol 1998;245:695–708 [DOI] [PubMed] [Google Scholar]
- 11.Lamy C, Oppenheim C, Meder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 2004;14:89–96 [PubMed] [Google Scholar]
- 12.Kinoshita T, Moritani T, Shrier DA, et al. Diffusion-weighted MR imaging of posterior reversible leukoencephalopathy syndrome: a pictorial essay. Clin Imaging 2003;27:307–315 [DOI] [PubMed] [Google Scholar]
- 13.Nelson RW, Frank JT. Intrathecal methotrexate-induced neurotoxicities. Am J Hosp Pharm 1981;38:65–68 [PubMed] [Google Scholar]
- 14.Tuxen MK, Hansen SW. Neurotoxicity secondary to antineoplastic drugs. Cancer Treat Rev 1994;20:191–214 [DOI] [PubMed] [Google Scholar]
- 15.Bernini JC, Fort DW, Griener JC, et al. Aminophylline for methotrexate-induced neurotoxicity. Lancet 1995;345:544–547 [DOI] [PubMed] [Google Scholar]
- 16.Drachtman RA, Cole PD, Golden CB, et al. Dextromethorphan is effective in the treatment of subacute methotrexate neurotoxicity. Pediatr Hematol Oncol 2002;19:319–327 [DOI] [PubMed] [Google Scholar]
- 17.Romero JM, Schaefer PW, Grant PE, et al. Diffusion MR imaging of acute ischemic stroke. Neuroimaging Clin N Am 2002;12:35–53 [DOI] [PubMed] [Google Scholar]
- 18.Peyriere H, Poiree M, Cociglio M, et al. Reversal of neurologic disturbances related to high-dose methotrexate by aminophylline. Med Pediatr Oncol 2001;36:662–664 [DOI] [PubMed] [Google Scholar]