Abstract
BACKGROUND AND PURPOSE: Previous studies have retrospectively reported the positive effects of percutaneous vertebroplasty. The purpose of our study was to evaluate prospectively the effects of vertebroplasty on mobility, analgesic use, pain, and SF-36 (short-form 36-item) scales for patients with painful vertebral compression fractures that are refractory to medical therapy.
METHODS: We prospectively followed 167 patients who received 207 vertebroplasty treatment sessions for stabilization of 264 symptomatic vertebral compression fractures between August 1999 and January 2003. The average age of patients was 74.6 years (SD = 12.2 years), and 76% were women. Pre- and postprocedural measurements of pain, mobility, analgesic use, and SF-36 scales were compared at 1 month after the procedure and between 6 months and 3 years after the procedure with the SF-36 scales.
RESULTS: Respective pre- and post-treatment pain scores were 8.71 (SE = 0.1) and 2.77 (SE = 0.18; P < .00001). Respective pre- and post-treatment analgesic use scores were 2.93 (SE = 0.9) and 1.64 (SE = 0.09; P < .00001). Respective pre- and post-treatment activity levels were 2.66 (SE = 0.1) and 1.64 (SE = 0.11; P < .00001). There was a statistically significant improvement on nine of 10 SF-36 scales (P < .001) after 1 month and on eight of 10 SF-36 scales (P < .02) at long-term follow-up.
CONCLUSION: Percutaneous vertebroplasty offers statistically significant benefits in decreasing pain, decreasing use of analgesics, and increasing mobility in appropriately selected patients. Percutaneous vertebroplasty also offers a statistically significant benefit in most SF-36 scales at both short- and long-term follow-up.
Vertebral compression fracture associated with osteoporosis is an increasingly common problem, with approximately 700,000 osteoporotic related vertebral fractures occurring each year (1). Symptomatic vertebral fractures currently account for >150,000 hospitalizations, 161,000 physician office visits, more than five million restricted activities days annually among Americans >65 years of age and affect 25% of postmenopausal women (2, 3). The lifetime risk of symptomatic vertebral fracture is 16% for white women and 5% for white men (2). It is estimated that the severity of osteoporosis and its clinical consequences such as hip and other fragility fractures will increase fourfold in the next half century because of increases in worldwide population and longevity (1).
Traditional medical therapy of vertebral body compression fractures includes analgesics, immobilization, muscle relaxants, physical therapy, and the use of external bracing. Percutaneous vertebroplasty has emerged as a viable alternative, especially in those patients who are refractory to conservative treatment. Since its introduction, there has been multiple case series in the medical literature reporting high success rates with percutaneous vertebroplasty in the treatment of vertebral compression fractures related to various etiologies (2, 4–11). More recent studies have retrospectively evaluated the short-term benefits of percutaneous vertebroplasty on various functional scales and have prospectively evaluated the short-term benefit of percutaneous vertebroplasty on pain scale (12–14).
Our study prospectively evaluated whether percutaneous vertebroplasty offered a statistically significant improvement on mobility, analgesic use, and pain scale for patients with painful osteoporotic vertebral compression fractures that are refractory to medical therapy. Our study also prospectively evaluated whether percutaneous vertebroplasty offered any statistically significant improvement on various SF-36 (short-form 36-item) scales.
Methods
Patient Population
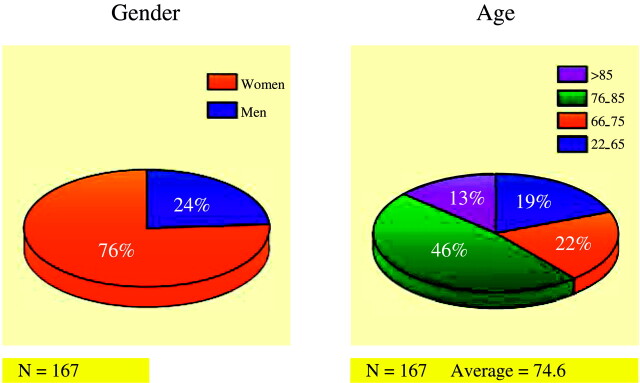
The average age of patients was 74.6 years (SD = 12.2 years). Seventy-six percent (127/167) of the patients were women, and 24% (40/167) were men (Fig 1). A total of 167 patients were enrolled in the study, with 35 (21%) patients receiving two or more vertebroplasty treatment sessions to comprise a total of 207 vertebroplasty treatment sessions (Fig 2).
Fig 1.
Patient demographics, by gender and age.
Fig 2.
Left, Percentage of vertebroplasty treatment sessions for one, two, and three fractures. Right, Percentage of patients receiving one, two, or three or more treatment sessions.
Patient Selection
All patients had osteoporotic compression fractures that were refractive to medical therapy, which consisted of a combination of bed rest, pain medication, muscle relaxant medication, external braces, and physical therapy. All patients had imaging evidence of compression fracture(s) with pain localized to the fracture level(s) and presented within 4 months of the fracture (range, 2 weeks to 4 months). Although we would entertain treating patients with more chronic fractures, none of our patients had symptomatic fractures that were older than 4 months. Disqualifying conditions included patients with pain thought to be due to herniated disks and/or spinal stenosis, fractures that responded to medical therapy, and the presence of any systemic or spinal infection.
Percutaneous Vertebroplasty Procedure
Percutaneous vertebroplasty procedures were performed at our institution and done in a consistent manner. The patient was placed in a prone position on an angiography table and 1 g of prophylactic intravenous cephazolin was given 30 minutes before the procedure (15). The fractured vertebral body was isolated on both true anteroposterior (AP) and lateral planes. The pedicle was isolated on the lateral plane for positioning in the inferior superior plane and under AP oblique fluoroscopy for a lateral to medial approach. Conscious sedation with intravenous fentanyl and midazolam and local anesthesia with 0.25% bupivacaine were administered followed by placement of an 11- or 13-gauge needle with an inner stylet (Parallax Medical/ArthroCare, Sunnyvale, CA), which was then advanced through the pedicle into the vertebral body under biplanar fluoroscopic guidance. Targeted placement of the needle tip was in the anterior third of the vertebral body near or across the midline. Polymethylmethacrylate (PMMA) was then prepared and injected by using a vertebroplasty injection system (Parallax/ArthroCare). Before 2001, patients were treated with Codman Cranioplastic, type 1 (slow setting) PMMA (CMW Laboratories, Blackpool, England), and sterile barium sulfate powder (EZ-EM, Westbury, NY). Since 2001, patients were treated with Secour Vx PMMA and TRACERS sterile barium opacifying agent (Parallax/ArthroCare). After the procedure, the patients were asked to remain supine for 2 hours to allow for complete curing of the PMMA and for the anesthesia to wear off. All patients were seen and examined by a member of the treating team and deemed medically stable before discharge.
Data Collection
Between August 1999 and January 2003, 167 patients had 207 vertebroplasty treatment sessions to treat 264 painful vertebral compression fractures. Before the procedure, institutional review board approval and patient informed consent were obtained. Pain, mobility, and analgesic use scores were obtained from all patients by phone, mail, or during clinic visits before receiving vertebroplasty. Information on pain, mobility, and use of analgesics was obtained from between 138 and 143 (83%–86%) patients 1 month after the procedure depending on the scale.
The visual analog pain scale was scored from 0 to 10, with 0 representing no pain and 10 representing the worst pain in the patient’s life. Mobility scale was scored from 0 to 5, with 0 representing full activity, 1 walking with assistance, 2 walking with assistance for short periods, 3 walking with assistance for activities of daily living/appointments only, 4 confined to a wheelchair when upright, and 5 bedridden. Analgesic use scale was scored from 0 to 5, with 0 representing no analgesic use, 1 aspirin, Tylenol, nonsteroidal anti-inflammatory drugs, 2 prescription non-narcotics, 3 oral narcotic as needed, 4 oral narcotic scheduled, and 5 parental narcotic.
The SF-36 scale used in our study is a 36-item questionnaire that has been used in clinical practice, research, health policy evaluations, and general population surveys. The SF-36 scale assessed 10 health scales, including (1) limitations on physical activities because of health problems (physical-functioning scale); (2) limitations on social activities because of physical or emotional problems (social-functioning scale); (3) limitations on usual role activities because of physical health problems (role physical scale); (4) bodily pain scale; (5) general mental health scale (psychological distress and well-being); (6) limitations in usual role activities because of emotional problems (role emotional scale); (7) vitality (energy and fatigue) scale; (8) general health perceptions (general health scale); (9) physical summary scale; and (10) mental summary scale.
Preprocedural SF-36 scale data were obtained from 106 (65%) of the 167 patients. Patients who were excluded included those who spoke limited English, who were not cognitively intact, or who refused to fill out the survey. One month after the procedure, SF-36 scale surveys were sent out by mail to those individuals who did not fall into the exclusion criteria stated above, and 92 (55.1%) survey responses were received. Between December 2002 and April 2003, SF-36 scale surveys were mailed again and 79 (47.3%) survey responses were received. By the time of long-term follow-up, 18 patients had died whereas no patients died by the time of short-term follow-up. None of these deaths was thought to be directly related to the vertebroplasty procedure. The long-term follow-up dates ranged from 6 months to as long as 3 years after the procedure. SF-36 scale data were in general more difficult to obtain than pain, analgesic use, and mobility scale data because of loss of contact information at long-term follow-up, death, language barriers, dementia, and patient ambivalence.
Statistical Analysis
Preprocedural and postprocedural measurements on pain, mobility, and analgesic use were compared by using multivariable statistical analysis. SF-36 scales were scored by using the SF-36 scale survey manual and interpretation guide.
Results
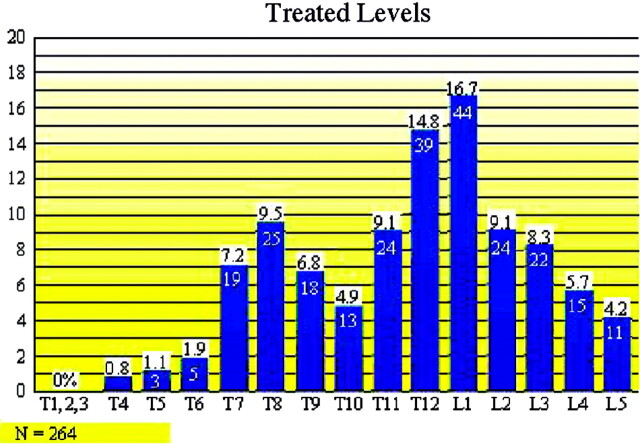
Vertebral compression fractures treated in our study extended from T4 to L5 (Fig 3). Our study found that T8 (n = 25), T12 (n = 39), and L1 (n = 44) were the most commonly fractured vertebral levels (108/264 [41%]). Seventy-three percent (151/207) of vertebroplasty treatment sessions involved treatment of only one symptomatic fracture. Twenty-five percent (52/207) of vertebroplasty treatment sessions involved treatment of two simultaneously symptomatic fractures. Thirty-two of 52 (62%) of these treatment sessions involved stabilization of adjacent fractures. Two percent of vertebroplasty treatment sessions (4/207) involved treatment of three symptomatic fractures (Fig 2). Seventy-nine percent (132/167) of patients required only one vertebroplasty treatment session. Seventeen percent (29/167) of patients needed a second vertebroplasty procedure to treat a fracture at either an adjacent or remote level. Eighteen (18/29 [62%]) of these patients had new fractures at an adjacent vertebral level, whereas 38% had a fracture separated by two or more vertebral levels from the original fracture. No patients experienced progressive compression of a previously treated fracture level. We did not find a statistically significant difference in terms of whether new fractures occurred above or below the previous fracture level. Three percent of patients (5/167) returned for two additional vertebroplasty treatment sessions for new fractures. One patient required five total vertebroplasty treatment sessions (Fig 2).
Fig 3.
Distribution of vertebral body fractures and treatment levels.
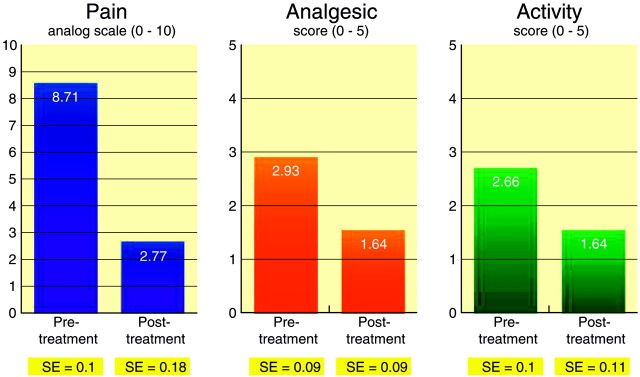
Pretreatment pain score average was 8.71 (SE = 0.1), whereas 1-month post-treatment pain score average was 2.77 (SE = 0.18; P < .00001; t = 28.97). Pretreatment analgesic use average was 2.93 (SE = 0.09), whereas 1-month post-treatment analgesic use average was 1.64 (SE = 0.09; P < .00001; t = 10.35). Pretreatment activity level average was 2.66 (SE = 0.1), whereas 1-month post-treatment activity level average was 1.46 (SE = 0.11; P < .00001; t = 9.13) (Fig 4).
Fig 4.
Graph comparing scores on pain and active scales prior to vertebroplasty and 1 month after vertebroplasty.
When comparing pre- and postvertebroplasty scores at 1-month follow-up on the 10 SF-36 health scales, we found improvement of 10.39 points (P < .0001) on the physical-functioning scale, improvement of 22.73 points (P < .0001) on the role physical scale, improvement of 25.97 points (P < .0001) on the bodily pain scale, improvement of 16.92 points (P < .0001) on the vitality scale, improvement of 23.72 points (P < .0001) on the social-functioning scale, improvement of 29.00 points (P < .0001) on the role emotional scale, improvement of 12.31 points (P < .0001) on the mental health scale, improvement of 3.36 points (P = .0007) on the physical summary measure scale, and improvement of 9.14 points (P < .0001) on mental summary score scale (Table 1). There was no statistically significant improvement on the general health scale. When comparing prevertebroplasty and long-term follow-up vertebroplasty scores on all 10 SF-36 health scales, we found improvement of 13 points (P = .0004) on the physical-functioning scale, improvement of 22.27 points (P < .0001) on the role physical scale, improvement of 23.58 points (P < .0001) on the bodily pain scale, improvement of 18.09 points (P < .0001) on the vitality scale, improvement of 19.32 points (P < .0001) on the social-functioning scale, improvement of 8.44 points (P = .0092) on the mental health scale, improvement of 5.50 points (P = .0005) on the physical summary measure scale, and improvement of 5.40 points (P = .0151) on the mental summary score scale (Fig 5). There was no statistically significant improvement on the general health scale and role emotional score scale.
Comparison of pre- and post-vertebroplasty treatment in 10 SF-36 health scale scores at 1-month and 6-month follow-up with quantitative values for changes in scores and P values
| Scale | Pretreatment |
1 Month |
PT–1-Month Change |
6 Months |
PT–6-Month Change |
P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| PF | 105 | 25.43 | 23.14 | 86 | 39.77 | 26.73 | 77 | 10.39 | 22.00 | 65 | 43.38 | 32.14 | 55 | 13.00 | 25.43 | 0.0004 |
| RP | 104 | 9.13 | 21.77 | 86 | 34.30 | 36.99 | 77 | 22.73 | 36.54 | 65 | 35.38 | 36.95 | 55 | 22.27 | 30.68 | <0.0001 |
| BP | 103 | 15.82 | 14.81 | 83 | 43.87 | 21.72 | 73 | 25.97 | 23.94 | 58 | 41.14 | 20.88 | 50 | 23.58 | 21.71 | <0.0001 |
| GH | 103 | 55.67 | 24.19 | 87 | 54.71 | 24.23 | 77 | −4.10 | 21.79 | 64 | 52.08 | 25.03 | 55 | −4.58 | 25.42 | 0.1869 |
| VT | 105 | 23.95 | 19.12 | 87 | 43.91 | 22.14 | 78 | 16.92 | 21.49 | 65 | 44.31 | 22.84 | 55 | 18.09 | 20.58 | <0.0001 |
| SF | 104 | 33.05 | 27.48 | 87 | 61.06 | 29.74 | 78 | 23.72 | 33.93 | 63 | 61.11 | 32.31 | 55 | 19.32 | 35.09 | <0.0001 |
| RE | 104 | 39.42 | 44.25 | 86 | 70.54 | 40.07 | 77 | 29.00 | 52.80 | 65 | 65.64 | 41.65 | 55 | 13.94 | 59.04 | 0.0856 |
| MH | 105 | 60.38 | 21.94 | 87 | 73.66 | 20.00 | 78 | 12.31 | 19.08 | 65 | 72.43 | 18.98 | 55 | 8.44 | 23.17 | 0.0092 |
| PCS | 102 | 28.16 | 7.06 | 82 | 32.99 | 8.82 | 71 | 3.36 | 8.02 | 57 | 33.72 | 11.87 | 50 | 5.50 | 10.44 | 0.0005 |
| MCS | 102 | 40.77 | 12.72 | 82 | 50.28 | 10.76 | 71 | 9.14 | 12.82 | 57 | 48.81 | 11.18 | 50 | 5.40 | 15.16 | 0.0151 |
Note.—PF indicates physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical summary score; MCS, mental summary score.
In summary, nine of 10 SF-36 health scales showed statistically significant improvement when comparing prevertebroplasty to 1-month follow-up scores, whereas eight of 10 SF-36 health scales showed statistically significant improvement when comparing prevertebroplasty to long-term follow-up scores. There were no major complications secondary to the vertebroplasty procedure. There was no worsening of pain, systemic infection, pulmonary embolism, or transient or permanent neurologic injury.
Discussion
Our study is unique in that it prospectively analyzed and showed in a large cohort the benefits of percutaneous vertebroplasty on mobility, pain, analgesic use, and SF-36 scales. Although there have been case series and retrospective studies evaluating the short-term benefits of percutaneous vertebroplasty (2, 4–14), few reports have attempted to evaluate any long-term benefits that vertebroplasty may offer (13, 16). Evans et al performed a retrospective multicenter study of 488 consecutive patients undergoing vertebroplasty of which 245 patients were interviewed comprising a 50% response rate (12). Their study currently provides the strongest support for the clinical benefits of vertebroplasty as they found statistically significant short-term benefits on pain, ambulation, and ability to perform daily activity scales. McGraw et al performed a smaller prospective study with 100 patients that showed benefits in pain scale, but did not evaluate any other scales (13). Zoarski et al prospectively evaluated the long-term outcomes of vertebroplasty in 30 patients by using a MODEMS (musculoskeletal outcomes data evaluation and management scale) and pain scale (16) and found statistically significant benefits on both scales. Our report, to our knowledge, is the largest prospective study that attempts to address long-term benefits of vertebroplasty with the use of a standardized SF-36 health scale in addition to short-term benefits with the use of SF-36 health, pain, analgesic, and mobility scales. Other strengths of our study include our high patient response rate and the prospective design of the study, creating less recall bias and adding further validity to the results of our report.
We found that T8, T12, and L4 were the most commonly fractured levels in our study. This observation is consistent with previous epidemiologic studies that have evaluated the incidence and clinical profile of osteoporotic vertebral compression fractures (17, 18). We found that 62% of vertebroplasty treatment sessions that treated two contemporaneous symptomatic fractures involved stabilization of fractures at adjacent levels in the primary treatment session. Similarly, in 62% of patients needing a second treatment session for new fractures, fractures occurred at an adjacent level. Thus, in our study, the rate of adjacent fractures was similar in the group of patients who presented with two fractures compared with patients who presented with new adjacent fractures after having received prior vertebroplasty. Our data are broadly consistent with prior studies, which suggest that percutaneous vertebroplasty does not increase the risk of adjacent vertebral fracture development (19, 20).
Although percutaneous vertebroplasty has emerged as a viable treatment option for osteoporotic vertebral compression fractures that are refractory to medical therapy, it is still important to be aware of the procedure’s potential clinical complications. When reviewing all major vertebroplasty series in well-trained hands, the clinical complication rate ranges from 1% to 10%, with osteoporotic patients having a complication rate of approximately 1%–3%, hemangioma patients having a 5% complication rate, and patients with metastases to the vertebra having a 10% complication rate (5). It should be stressed that, when well-trained physicians perform vertebroplasty to treat osteoporotic vertebral compression fractures, complication rates can be <1%. None of the 167 patients in our study had clinical complications following their vertebroplasty procedures. Potential clinical complications of percutaneous vertebroplasty that have been documented in case reports and case series in the medical literature include infection, bleeding, back pain, rib fracture, pulmonary embolism, pneumothorax from punctured lung, transient arterial hypotension, fever, optic neuritis, and various other neurologic complications (5, 7, 21–27).
One of the limitations of our study was that SF-36 scale data were difficult to obtain in many patients, because of the complexity of the questionnaire, language barriers, death, loss to follow-up, and patient apathy. We had a 56% overall response rate of SF-36 scale surveys. Our study’s 85%–90% overall response rate of pain, mobility, and analgesic use scales was significantly higher than the 50% response rate found in the Evans et al study that also used these scales (12). Because 11% of our patients had died at the time of long-term follow-up, it is possible that these patients did not gain as many long-term benefits as those patients that survived. Although patient deaths may have affected our long-term follow up data, they did not affect our short-term follow-up data because no patients died between the procedure and 1-month follow-up. Another potential limitation of this study was that we did not have a consistent time period for long-term SF-36 scale survey follow-up as the long-term follow-up time intervals varied from 6 months to 3 years. Nevertheless, this information can be used to extrapolate whether the beneficial effects of vertebroplasty decrease with time or whether the benefits remain relatively stable after 6 months. A final disadvantage of our study was that we did not have a medical treatment comparison group, which points to the need for a double-blinded randomized controlled trial. Some of our patients were treated after having received a trial of medical therapy by the referring primary physicians. Because the length of time of conservative therapy before vertebroplasty treatment can range from several weeks to several months, there was a lack of uniformity in the conservative treatment group in our study. As a result, this did not allow for our patients to serve as their own controls.
Conclusion
Our prospective study indicates that percutaneous vertebroplasty offers statistically significant benefits in decreasing pain, decreasing use of analgesics, and increasing mobility in appropriately selected patients. Vertebroplasty also offers statistically significant benefits on most SF-36 health scales, and these benefits do not appear to decrease with long-term follow-up, which suggests that this technique is durable and offers significant long-term benefit in appropriately selected patients.
References
- 1.Riggs BL, Melton LJ III. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995;17:505S–511S [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ III. Epidemiology of spinal osteoporosis. Spine 1997;22(suppl 24):2S–11S [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen SJ, Cooper C, Gottlieb MS, et al. Hospitalization with vertebral fracture among the aged: a national population based study, 1986–1989. Epidemiology 1992;3:515–518 [DOI] [PubMed] [Google Scholar]
- 4.Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525–530 [DOI] [PubMed] [Google Scholar]
- 5.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Radiol Clin North Am 1998;36:533–546 [DOI] [PubMed] [Google Scholar]
- 6.Weill A, Chiras J, Simon J, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology 1996;199:241–247 [DOI] [PubMed] [Google Scholar]
- 7.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897–1904 [PMC free article] [PubMed] [Google Scholar]
- 8.Cyteval C, Sarrabere MP, Roux JO, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol 1999;173:1685–1690 [DOI] [PubMed] [Google Scholar]
- 9.Debussche-Depriester C, Deramond H, Fardellone P. Percutaneous vertebroplasty with acrylic cement in the treatment of osteoporotic vertebral crush fracture syndrome. Neuroradiology 1991;33:149–1522046900 [Google Scholar]
- 10.Depriester C, Deramond H, Toussaint P. Percutaneous vertebroplasty: indications, technique, and complications. In: Connors JJ III, Wojak JC, eds. Interventional neuroradiology: strategies and practical techniques. Philadelphia: WB Saunders;1999. :347–357
- 11.Gangi A, Dietemann JL, Guth S, et al. Computed tomography (CT) and fluoroscopy-guided vertebroplasty: results and complications in 187 patients. Semin Interv Radiol 1999;16:137–142 [Google Scholar]
- 12.Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty: retrospective report of 245 cases. Radiology 2003;226:366–372 [DOI] [PubMed] [Google Scholar]
- 13.McGraw JK, Lippert JA, Minkus KD, et al. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol 2002;13:883–886 [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann TJ, Jensen JE, Schweickert PA, et al. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2001;22:1860–1863 [PMC free article] [PubMed] [Google Scholar]
- 15.Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–381 [PMC free article] [PubMed] [Google Scholar]
- 16.Zoarski GH, Snow PA, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002;13:139–148 [DOI] [PubMed] [Google Scholar]
- 17.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992;7:221–227 [DOI] [PubMed] [Google Scholar]
- 18.Patel U, Skingle S, Campbell GA, et al. Clinical profile of acute vertebral compression fractures in osteoporosis. Br J Rheumatol 1991;30:418–421 [DOI] [PubMed] [Google Scholar]
- 19.Jensen ME, Dion JE. Percutaneous vertebroplasty in the treatment of osteoporotic compression fractures. Neuroimaging Clin North Am 2000;10:547–568 [PubMed] [Google Scholar]
- 20.Jensen ME, Kallmes DF, Short JG. Percutaneous vertebroplasty does not increase the risk of adjacent vertebral fracture: a retrospective study. In: Proceedings of the ASNR, Atlanta, GA, April 3–8,2000
- 21.Gaughen JR, Jensen ME, Schweickert PA, et al. Relevance of antecedent venography in percutaneous vertebroplasty for treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol 2002;23:594–600 [PMC free article] [PubMed] [Google Scholar]
- 22.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine 2000;25:923–928 [DOI] [PubMed] [Google Scholar]
- 23.Padovani B, Kasriel O, Brunner P, Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:375–377 [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington KD. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg Am 2002;83:1070–1073 [DOI] [PubMed] [Google Scholar]
- 25.Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate; a case report. Spine 2002;27:419–422 [DOI] [PubMed] [Google Scholar]
- 26.Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine 2002;27:416–418 [DOI] [PubMed] [Google Scholar]
- 27.Vasconcelos C, Gailloud P, Martin JB, Murphy KJ. Transient arterial hypotension induced by polymethylmethacrylate injection during percutaneous vertebroplasty. J Vasc Interv Radiol 2001;12:1001–1002 [DOI] [PubMed] [Google Scholar]