Abstract
BACKGROUND AND PURPOSE: The best time for surgery after embolization of meningiomas remains unclear. We used the tumor-softening effect of embolization to determine this time.
METHODS: Forty-two patients with intracranial meningiomas that received more than 50% of their blood supply from the external carotid artery underwent embolization before surgery. The interval between embolization and surgery and the tumor consistency at the time of surgery were recorded. The interval between embolization and surgery was divided into 3-day segments, and an average tumor consistency score was obtained in segments. Patients were assigned to two groups: group 1 underwent surgery on a specified day after embolization; group 2 underwent surgery at a later date. We compared tumor consistency, blood loss, length of hospitalization, surgical resection time, Simpson grade at surgery, and complication rate in both groups.
RESULTS: On the polynomial regression curve, greatest tumor softening occurred 7–9 days after embolization. When the postembolization interval exceeded 10 days, no further softening occurred. Compared with group 1 patients, group 2 patients did not have an increased blood loss, a longer hospitalization, or a higher complication rate. In group 2, the surgical procedure required less time, and the Simpson grades were lower.
CONCLUSION: In meningiomas that receive more than 50% of their blood supply from the external carotid artery, the optimal interval between embolization and surgery is 7–9 days. This interval allows the greatest degree of tumor softening, which makes it possible to remove the tumor more safely and easily.
Advances in radiologic and catheterization techniques have enabled the application of endovascular procedures to various types of vascular lesions, including highly vascular meningiomas (1–3). Dean et al (4) described the clinical effectiveness of tumor embolization in patients with meningiomas. Although embolization decreases blood loss and reduces the need for intraoperative transfusions, the best timing of surgery after embolization remains unclear.
We retrospectively reviewed the clinical and imaging data in 65 patients who underwent preoperative embolization to determine the optimal interval between embolization and surgery. The aim was to acquire a tumor softening index.
Methods
Between July 1995 and March 1999, 65 patients with intracranial meningioma underwent preoperative particle embolization at our institution. The imaging and medical records of all 65 patients were retrospectively reviewed. All patients underwent conventional angiography with digital subtraction angiography (DSA) and bilateral selective catheterization of the external and internal carotid arteries. Two of the authors (M.M., T.T.) independently judged the ratio of the vascular supply derived from the internal or external carotid arteries or both. Tumor vascularity was determined as the degree of blush within the tumor on DSA images (anteroposterior and lateral views).
For this study, we selected 42 patients in whom the external carotid artery supplied more than 50% of the blood flowing to the tumor and in whom diffuse homogeneous contrast material accumulation persisted into the venous phase of angiography. Moreover, the diameter of the meningiomas was at least 4 cm on MR images or CT scans. Patients with meningiomas that demonstrated obvious calcification on CT scans were excluded. The patients included 27 women and 15 men with an age range of 17–73 years (mean, 54.4 years). Of their 42 tumors, seven were petroclival, four were occipital, one was parasagittal, 17 were at the sphenoidal ridge, five were at the convexity, four were in the middle fossa, three were in the cavernous sinus, and one was in the falx.
In all 42 patients, results of the preembolization provocation test with xylocaine (usually 40 mg) were negative. DSA and preoperative embolization were performed via the transfemoral approach with the patients under local anesthesia. A microcatheter (Tracker 18; Target Therapeutics, San Jose, CA) was introduced into the proper feeding arteries. The embolization particles were cellulose porous beads (200 μm), nonabsorbable particles that were developed in our department (5–7).
The microneurosurgical procedures involved excavation of the central tumor mass by using suction or a Cavitron ultrasonic surgical aspirator (CUSA Sonopet UST-2000; M&M, Tokyo, Japan) or by cutting with scissors to completely remove the tumor. The senior author (Y.U.) reported consistency of the tumor during surgery and recorded microscopic findings.
Tumor consistency was divided into three categories and scored as follows: score 3 was used for tumors of soft consistency that allowed the removal of more than 50% of the tumor volume with suction or low-power (10–30%) ultrasonic surgical aspiration; score 2, tumors of moderately hard consistency that allowed the removal of more than 50% of the tumor mass with high-power (40–100%) ultrasonic surgical aspiration; and score 1, hard tumors for which scissors were used to resect more than 50% of the tumor volume.
The interval between embolization and surgery was divided into 3-day segments, and the average score (±SD) of tumor hardness at the time of surgery was calculated for each segment. The mean scores in each segment were plotted for each 3-day segment, and the polynomial regression curve was obtained by using the multiple regression procedures from commercial software. In all 42 patients with intracranial meningioma, the optimal day for postembolization surgery was determined from this curve.
According to the polynomial regression curve, we set an optimal day after embolization for surgery. The patients were then assigned to two groups: those who underwent surgery on the optimal day were in group 1, and those who underwent surgery on a later day were in group 2. In both groups we calculated the mean tumor consistency score (±SD) at the time of surgery.
To determine whether the waiting period after embolization had any negative effects, we recorded blood loss during surgery and the length of hospitalization in the two groups. To assess whether embolization-induced tumor softening contributed to the ease of tumor removal, we also recorded the length of time required for the surgical procedures and the Simpson grade; we compared the results obtained in the two groups.
The patients’ clinical course after embolization was defined as asymptomatic or symptomatic. The clinical outcomes were evaluated by reviewing the charts and determining the Karnofsky Performance Status (KPS) 3 months after surgery (8).
The Mann-Whitney U test was used to determine the statistical significance of the difference between the two groups. Statistical significance was defined when the P value was less than .05.
Results
Of the 42 patients who underwent surgery, 13 had hard tumors, 24 had tumors of moderately hard consistency, and the remaining five had soft tumors. In this series, the interval between tumor embolization and surgery was 1–25 days (eight 3-day segments).
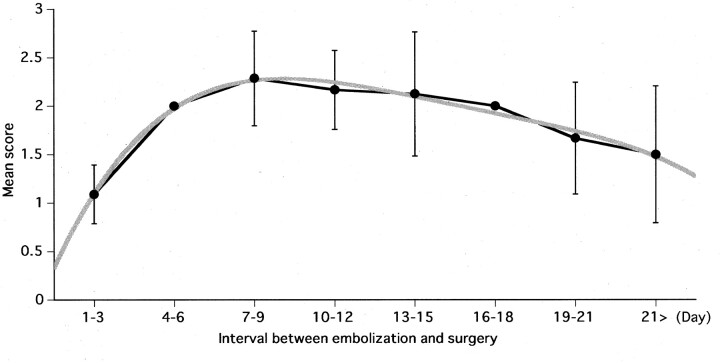
The mean score (±SD) in each of the 3-day segments after embolization was plotted, and the polynomial regression curve was drawn (Fig 1). Curve fitting by this regression analysis was high (R2 = .98), and the data clearly fit. The curve inclined steeply until the third 3-days segment (days 7–9 after embolization); the mean score slowly decreased thereafter. The greatest tumor softening effect was recorded in the 7–9-day segment after embolization.
Fig 1.
Curves show the values that represent the mean score (±SD) of tumor consistency at surgery for each 3-day segment after embolization. The polynomial regression curve of multiple regression procedures was overwritten (R2 = .98).
Of the 42 meningiomas, 21 were meningothelial, four were fibroblastic, nine were transitional, four were angiomatous, one was microcystic, and three were atypical. When surgery was performed 7–9 days after embolization, no correlation between the tumor subtype and the degree of softening was found.
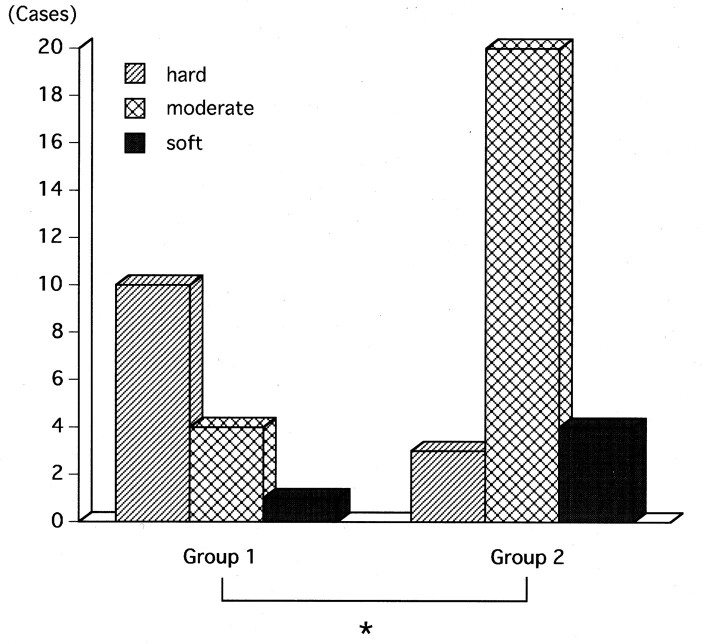
We selected the seventh day after embolization as the benchmark and analyzed our results by assigning the 42 patients into two groups: those who underwent surgery within 7 days of embolization (group 1, n = 15; range, 1–7 days; mean, 2.5 days) and those who underwent surgery more than 7 days after embolization (group 2, n = 27; range, 8–25 d; mean, 13.8 d). Group 1 patients had 10 hard and four moderately hard tumors; in one patient, the tumor consistency was soft. Group 2 patients had three hard, 20 moderately hard, and four soft tumors. The difference with respect to tumor hardness between the two groups was significant (P < .01) (Fig 2).
Fig 2.
Bar graph shows tumor consistency at surgery (hard, moderately hard, soft) for the two groups (group 1 interval between embolization and surgery, 1–7 days; group 2 interval, >7 days). * indicates P < .01.
The significant difference between the two groups persisted until days 8 or 9 (P = .02, P = .04) and disappeared when 10 or more days had elapsed between embolization and surgery (P = .27). Thus, an increased interval of more than 10 days resulted in no additional benefits in terms of the tumor softening effect.
When the seventh day after embolization was used to assign the patients to two groups, the results in group 1 versus those in group 2 were as follows: estimated blood loss, 739 versus 951 mL, and length of hospitalization, 20.7 versus 27.6 days. The differences in these variables were not statistically significant (P = .32 and P = .11, respectively).
The duration of the surgical procedure in group 1 was 534.6 minutes; in group 2, it was 435.0 minutes (P = .05). The Simpson grades at the surgery were 2.60 and 1.91, respectively, in group 1 and group 2 (P = .03).
Each group had one symptomatic patient. The symptomatic patient in group 1 had an intratumoral hemorrhage; the symptomatic patient in group 2 had hearing loss after embolization. No patient had transient postembolization complications. The KPS at 3 months after surgery in group 1 was 94.0; in group 2, it was 98.3 (P = .04).
Discussion
Embolization before surgery was beneficial in patients with large meningiomas because it decreased the blood loss and reduced the need for intraoperative transfusion (4). However, no general agreement about the optimal interval between tumor embolization and surgery in patients with meningiomas currently exists. Djindjian et al (9) recommended an interval of 3 days, and Brismar and Conqvist (10) suggested that surgery should occur 1 or 2 days after embolization. Their use of absorbable embolic materials, Gelfoam or Sponger, necessitated surgery after a short interval to prevent recanalization. The interval between embolization and surgery can be prolonged when new embolic materials such as silicone or acrylic polymers are used; this delay may maximize the effect of devascularization (1). In comparison, the use of our cellulose porous beads facilitated more distal, complete, and permanent occlusion of the feeding artery (5–7). Therefore, our recanalization rate was extremely low, and the interval between embolization and surgery could be prolonged to obtain the maximum benefits of the resultant tumor devascularization.
Dean et al and Teasdale et al (3, 11) observed no obvious relationship between the histologic type of the meningioma and the interval between embolization and surgery. However, as the interval between embolization and surgery increased, so did the degree of tissue reactions (12) and tumor softness, as well as the ease of tumor removal (2, 13).
Dean et al (4) found no significant increase in the length of hospitalization or in the severity of complications in patients with meningioma who underwent preoperative embolization. While the 7–9-day waiting period after embolization increased the overall length of hospitalization in patients with meningioma, it did not increase the incidence or severity of complications. If the additional days of hospitalization represent a hardship for patients, they could be temporarily discharged after embolization and readmitted for surgery.
Neither blood loss during surgery nor the risk of complications was increased because of the postembolization waiting period. In patients with embolization-induced tumor softening, removal of the tumor was easy and could be accomplished without injury to adjacent vital structures. The difference in surgical outcomes between the two groups was statistically significant: in group 2, less time was required for the surgical procedures than in group 1. In addition, the Simpson grade was lower in group 2 patients, and their KPS was better. Our data indicated that an interval of 7–9 days between embolization and surgery led to optimal treatment outcomes because of marked tumor softening. The beneficial effect of embolization was not further enhanced by prolonging the interval past 10 days.
Time-dependent proton spectra of embolized meningiomas depicted a large lactate signal during the first 24 hours after embolization; this was indicative of tissue ischemia (14, 15). Within 4 days, the lactate signal was replaced by broad aliphatic signals, which indicated tissue necrosis. These observations led to the conclusion that these signals were notable and should be used to determine the optimal timing of surgery after tumor embolization. Our findings supported the suggestion that a certain amount of time should be allowed to elapse between embolization and surgery, and they indicated that the greatest tumor softening effects were achieved when this interval was 7–9 days. Jungling et al (14) recommended that the waiting period should be longer than 5 days. This slight difference between their recommendation and our suggested optimal time interval may be attributed to the fact that we selected meningiomas that were at least 4 cm in diameter and that received more than 50% of their blood supply from the external carotid artery.
At this time, we do not know why waiting for more than 10 days after embolization did not further enhance its beneficial effects. We speculate that necrosis does not progress further after 7–9 days after embolization. Ng et al (3) reported that no obvious relationship between histologic changes and the postembolization interval existed. However, the process of coagulative necrosis has been documented in ischemic injury, and the degree of necrotic change tends to increase with time after ischemia (2, 12, 13). Necrosis after an ischemic insult is characterized by the presence of increasing numbers of inflammatory cells and by softening of the necrotic tissue due to autolysis. With progression of necrosis, a salvage process involving residual cellular debris occurs until, finally, the necrotic process ceases. We speculate that by 10 days after embolization, the necrotic changes have halted, and for this reason, no further tumor softening effects occur.
We scored the consistency of the tumor at the time of surgery. Judging the consistency on the basis of the appearance of nonenhanced areas on postembolization MR or CT images is difficult. We found that even nonenhanced lesions could become necrotic lesions (data not shown).
In our series, the meningiomas received more than 50% of their blood supply from the external carotid artery and were at least 4 cm in diameter, without obvious calcification. In patients with these meningiomas, we expected embolization to have a marked effect on the consistency of the meningioma. Further studies are needed to determine the optimal interval between embolization and surgery in patients whose tumors derive less than 50% of their supply from the external carotid artery.
Conclusion
Our results suggest that embolization has a softening effect on meningiomas and that the optimal interval between embolization with nonabsorbable materials and surgery is 7–9 days. Prolonging the interval had no further beneficial effects.
Acknowledgments
We are grateful to Hirofumi Shinohara for statistical analysis of our data.
References
- 1.Hieshima GB, Everhart FR, Mehringer CM, et al. Preoperative embolization of meningiomas. Surg Neurol 1980;14:119–127 [PubMed] [Google Scholar]
- 2.Koike T, Sasaki O, Tanaka R, Arai H. Long-term results in a case of meningioma treated by embolization alone: case report. Neurologia Medico Chirurgica 1990;30:173–177 [DOI] [PubMed] [Google Scholar]
- 3.Ng HK, Poon WS, Goh K, Chan MS. Histopathology of post-embolized meningiomas. Am J Surg Path 1996;20:1224–1230 [DOI] [PubMed] [Google Scholar]
- 4.Dean BL, Flom RA, Wallace RC, et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol 1994;15:1675–1680 [PMC free article] [PubMed] [Google Scholar]
- 5.Connors JJ III, Wojak JC. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia, Pa: W.B. Saunders;1999;28
- 6.Hamada J, Kai Y, Nagahiro S, Hashimoto N, Iwata H, Ushio Y. Embolization with cellulose porous beads, II: clinical trial. AJNR Am J Neuroradiol 1996;17:1901–1906 [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada J, Ushio Y, Kazekawa K, Tsukahara T, Hashimoto N, Iwata H. Embolization with cellulose porous beads, I: an experimental study. AJNR Am J Neuroradiol 1996;17:1895–1899 [PMC free article] [PubMed] [Google Scholar]
- 8.Simoca I, Olarescu AA, Jipescu I, Lisievici M. Postoperative outcome of intracranial meningiomas: long-term prognosis. Rom J Neurol Psychiatry 1994;32:237–251 [PubMed] [Google Scholar]
- 9.Djindjian R, Merland JJ, Rey A, Thurel J, Houdart R. Super-selective arteriography of the external carotid artery. Importance of this new technic in neurological diagnosis and in embolization. Neuro Chirurgie 1973;165–171 [PubMed]
- 10.Brismar J, Conqvist S. Therapeutic embolization in the external carotid artery region. Acta Radiol Diagn 1978;19:715–731 [DOI] [PubMed] [Google Scholar]
- 11.Manelfe C, Lasjaunias P, Ruscalleda J. Preoperative embolization of intracranial meningiomas. AJNR Am J Neuroradiol 1986;7:963–972 [PMC free article] [PubMed] [Google Scholar]
- 12.Richter HP, Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery 1983;13:261–268 [DOI] [PubMed] [Google Scholar]
- 13.Teasdale E, Patterson J, McLellan D, Macpherson P. Subselective preoperative embolization for meningiomas: a radiological and pathological assessment. J Neurosurg 1984;60:506–511 [DOI] [PubMed] [Google Scholar]
- 14.Jungling FD, Wakhloo AK, Hennig J. In vivo proton spectroscopy of meningioma after preoperative embolization. Magn Res Med 1993;30:155–160 [DOI] [PubMed] [Google Scholar]
- 15.Tymianski M, Willinsky RA, Tator CH, Mikulis D, TerBrugge KG, Markson L. Embolization with temporary balloon occlusion of the internal carotid artery and in vivo proton spectroscopy improves radical removal of petrous-tentorial meningioma. Neurosurgery 1994;35:974–977 [DOI] [PubMed] [Google Scholar]