Abstract
BACKGROUND AND PURPOSE: Carotid fusiform aneurysms are most commonly treated with occlusion of the parent vessel. The purpose of our study was to assess the effectiveness of self-expanding, cobalt-alloy stents in the ablation of experimental fusiform aneurysms with preservation of the parent vessel in a carotid artery model.
METHODS: Porous metallic stents were placed endovascularly along the lengths of experimentally created fusiform aneurysms in the carotid arteries of dogs; aneurysms were also created in the animals' opposite carotid arteries to serve as controls.
RESULTS: Before stent placement, angiography of the carotid arteries showed large fusiform aneurysms along the lengths of the common carotid arteries and complex patterns of flow. Immediately after stent placement there was disruption of the usual flow patterns within the lumens of the fusiform aneurysms. The lumen between the wall of the aneurysm and stented carotid showed stasis of contrast material and blood. Near-complete ablation of all aneurysms was observed 8 weeks after stent placement. The stented carotid arteries remained widely patent; control aneurysms and carotid arteries were patent and unchanged. Histopathologic analysis revealed fibrotic reactive scar tissue filling the space between the stent wires and outer wall of the fusiform aneurysm.
CONCLUSION: Changing blood flow dynamics within an aneurysm can promote thrombus formation. The stent promotes stasis and thrombus within the residual lumen between the stent wall and the outer wall of the aneurysm because its woven wire mesh interferes with usual blood flow patterns, which then promotes formation of thrombus and fibrosis within the residual aneurysmal lumen.
Fusiform aneurysms, unlike saccular aneurysms, are not amenable to endovascular therapies if associated preservation of the native vessel is desired. Current treatment of fusiform aneurysms consists of occlusion of the parent vessel or surgical resection of the aneurysm with graft replacement; this is associated with a high rate of morbidity and mortality (1, 2). Aneurysms near the skull base (eg, petrous or cavernous internal carotid artery aneurysms) are surgically inaccessible; therefore, the only option is vascular occlusion. Porous metallic stents are used to reopen vessels narrowed by atherosclerotic plaques (3, 4). Prior experiments with these stents in the treatment of experimental saccular aneurysms have had favorable results (4). The structured architecture of the stents' wire struts alters flow patterns within the aneurysmal pouch, which promotes organized thrombus formation and eventual ablation of these aneurysms. The purpose of our study was to determine the effectiveness of intravascular stents in the treatment of experimentally created fusiform aneurysms in canine carotid arteries. The tubular wire mesh of the porous stent reestablishes the original caliber of the native vessel while functionally excluding the aneurysmal compartment from the vessel lumen.
Methods
Experimental fusiform aneurysms were created in five mongrel dogs, each weighing about 30 kg. The hospital's institutional animal care and use committee approved our protocol.
The aneurysms measured 1.0 to 1.5 cm in length. The stents (Wallstents, Schneider, Minneapolis, MN) were porous, metallic, and 5 mm in diameter. They were placed in the carotid artery across the lengths of the fusiform aneurysms. The opposite carotid artery acted as a control. Aspirin was administered orally at a dosage of 10 mg/kg twice a day. Sonographic examination of the carotid arteries, including the aneurysms, was performed twice weekly. Angiography was performed before stent placement, immediately after deployment of the stents, and 8 weeks after stent placement.
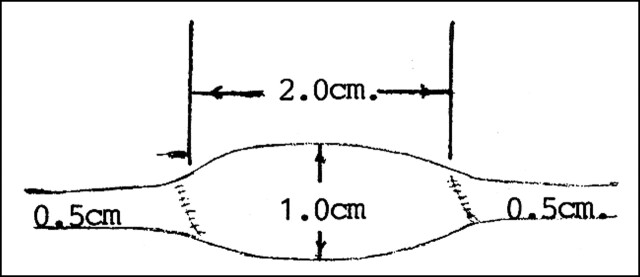
Creation of Fusiform Aneurysms (Fig 1)
fig 1.
Diagram of a fusiform carotid artery aneurysm from a jugular vein segment
Animals were anesthetized with sodium pentobarbital (30 mg/kg IV), endotracheally intubated, surgically prepped, and placed in the supine position. A 7-cm longitudinal, mid-cervical incision was made, but off midline to facilitate exposure of the left external jugular vein and left common carotid artery. The jugular vein was sufficiently mobile to allow a 4-cm segment to be excised. The free segment of vein was flushed with heparinized saline and placed in a saline bowl. A length of the left common carotid artery was mobilized to permit total occlusion with vascular clamps; it was then transected and a segment excised for the interposition of the free vein graft. The ends of the venous segment were tapered to permit anastomosis with the smaller caliber artery. A 2-cm-long vein graft was interposed into the carotid artery. The diameter ratio of vein to artery was 2:1. Vascular anastomosis was completed with 6–0 Surgilene (U.S. Surgical, Norwalk, CT). The wound was closed in layers with 4–0 Maxon (U.S. Surgical), terminating in a running subcuticular closure of the skin.
Histologic Process and Photography
The process by which the stented fusiform aneurysmal specimens were prepared for micropathologic examination has been described previously (5). After the specimens were processed and embedded, the paraffin blocks were cut into sections approximately 5-μm thick. Three slides were created from each section and the slides from each section were stained one each with hematoxylin-eosin, Masson's trichrome stain, and Verhoeff's elastin stain, respectively.
Stent Procedure
After the fusiform aneurysms were created, the dogs were allowed to heal for 1 week before arteriography was performed. Angiography was performed via the femoral artery approach. A cut-down procedure on the femoral artery was initially performed followed by insertion of a 7F introducer catheter. The common carotid arteries were selectively catheterized with conventional 5F cerebral catheters. A single investigator in this study with 15 years of angiographic experience interpreted all angiograms. At the end of this diagnostic study, the 5F catheter was exchanged for a 7F stent delivery system using an exchange guidewire. A single porous metallic stent (Wallstent) was deposited intravascularly into the carotid artery so that it covered the length of the fusiform aneurysm. Arteriography was performed immediately following and 8 weeks after stent deployment. The specimen was resected 8 weeks after stent placement.
The metallic wire of the stent is made of Elgiloy (Elgiloy Ltd Partnership, Elgin, IL), a nonmagnetic, corrosion-resistant, cobalt-chromium-iron-molybdenum alloy with a biocompatibility that makes it suitable for surgical instruments and implants. The Elgiloy wire was braided to construct the stent and then treated with heat to enhance the tensile and fatigue strength of the wire braid. The diameter of the wire is 0.035 inches, and the stent measures 42 mm in length by 5 mm in diameter unconstrained. The best results were achieved by oversizing the stent by approximately 1 mm more than the diameter of the native vessel (3). Since the native carotid artery of the dog has a diameter of approximately 4 mm, a stent with a 5-mm diameter was chosen. The stent was mounted on a delivery system consisting of coaxial catheters. The exterior tube served to constrain the stent until it was retracted during delivery (rolling membrane). The interior tube with a coaxial system contained a central lumen that can accommodate a 0.038-inch guidewire.
Results
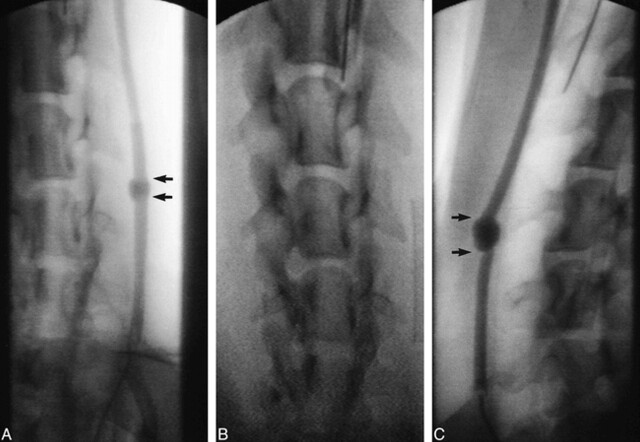
Angiographic Findings (Fig 2)
fig 2.
Angiographic findings of a fusiform carotid artery aneurysm.
A, Eight weeks after stent placement there is near-complete ablation of the aneurysmal lumen (arrows).
B, The Wallstent within the carotid artery.
C, The control fusiform aneurysm (arrows) and the carotid artery remain widely patent throughout the 8-week period of observation.
Immediately after stent deployment, flow between the stent and the aneurysmal wall was disturbed to a degree that stagnation was observed. In all cases there was near-complete obliteration of the aneurysmal pouch 8 weeks after stent placement. Small pockets of residual lumen were identified in all aneurysms 8 weeks after stent deployment. The control aneurysms remained widely patent and unchanged throughout the 8-week period. The carotid arteries remained widely patent. No focal stenosis was seen. The stented arteries, in particular, were widely patent without evidence of stenosis.
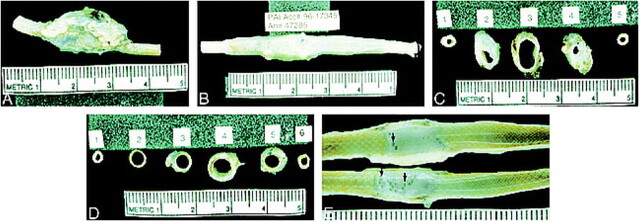
Gross Observation (Fig 3)
fig 3.
Gross pathologic examination.
A, Gross end-block specimen of control aneurysm shows the walls remain widely dilated.
B, The outer walls of the stented aneurysm have contracted along the length of the stent, producing a narrower profile of the carotid artery as compared with the profile of the control aneurysm.
C, Control aneurysm, cross section. Section 3 shows the markedly dilated lumen of the fusiform aneurysm.
D, Stented aneurysm. Section 4 is through the center of the fusiform aneurysm. Note the contraction of the outer walls of the aneurysm along the stent, again producing a narrower profile of the carotid artery and aneurysm as compared with the control aneurysm. The lumen between the stent and outer wall of the aneurysm is filled with white, organized thrombus.
E, Longitudinal section. All stents were embedded in a glistening, translucent neointima. The stented aneurysms appear solid except for small channels, which connect the lumens of the carotid arteries with those of the aneurysms (arrows).
The diameter of the control aneurysms was considerably larger than that of the stented aneurysms (Fig 3). The walls of the stented aneurysms were contracted along the outer wall of the stent wires, and the stent wires were embedded in a firm translucent neointima. The stented aneurysms appeared solid except for small channels connecting to the carotid artery lumen.
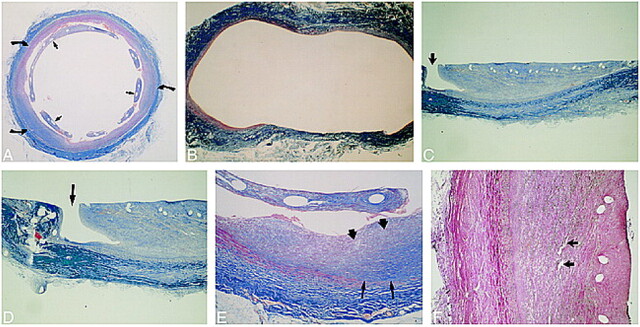
Histopathologic Findings (Fig 4)
fig 4.
Histopathologic findings.
A, Mason's trichrome stain on stented aneurysm shows the aneurysmal lumen is nearly completely filled in with organized fibrous connective tissue (curved arrows). The stent wires are surrounded by neointima (straight arrows).
B, Mason's trichrome stain on control aneurysm in cross section shows markedly distended thin wall of the aneurysm.
C and D, Trichrome stain on stented aneurysm in longitudinal section shows a small, patent endothelial-lined channel at one margin, connecting the carotid lumen with the aneurysmal lumen (arrow). These small channels were seen in all aneurysmal specimens. The remainder of the aneurysmal lumens were filled in by fibrous connective tissue (organized thrombus). Within the neointima surrounding the stent wires there was mild inflammatory cell infiltration (macrophages). Many macrophages contained blood pigment (resolving thrombus).
E, Magnified cross-sectional view of aneurysmal lumen with Mason's trichrome stain. Fibrin and collagen stain blue. Dark blue represents more mature fibrin and collagen. Mature fibrous elements and collagen (dark blue) were noted near the periphery of the aneurysmal wall (thin arrows); less mature fibrous elements (light blue) lie near the central lumen of the aneurysm (thick arrows), suggesting that thrombus organization begins along the outer wall and progresses in a centripetal fashion toward the central lumen of the aneurysm.
F, Verhoeff-van Gieson elastic stain shows heavily vascularized organized thrombus (arrows).
In all the stented samples, the aneurysms were filled with mature fibrous connective tissue (organized thrombus) except for occasional small (400-μm wide) irregular channels that connected to the vessel lumen (Fig 4C and D). While no immunohistochemical preparations (eg, factor VIII or lectins) were made, the small channels and several small spaces within the fibrous tissue (Fig 4F) were lined by cells morphologically indistinguishable from the endothelial cells that lined the luminal surface of the nonstented sections and the cells that covered the fibrous tissue within which the stent was embedded. These lining cells were continuous with the lining cells (endothelium) of the lumen. The small spaces within the fibrous tissue were expanded neocapillaries (Fig 4F). Because of the way thrombi heal, these neocapillaries are present within the fibrous tissue. They often expand widely as the fibrous tissue matures and contracts. Fibrous tissue (like any other tissue) needs nutrients to exist. Within the fibrous connective tissue (deep to the organized mural thrombus or neointima surrounding the stent wires), there was mild inflammatory cell infiltration consisting primarily of macrophages, many of which contained hemosiderin. The inflammatory cell infiltrate (macrophages, many of which were hemosiderin-laden) was typical of a maturing (organizing) blood clot. First the fibrin holds the blood cells together, then inflammatory cells come in to phagocytize the residual blood cells and detritus while fibroblasts migrate in to proliferate and secrete collagen. The macrophages have hemosiderin in them because they have phagocytized the degenerate blood cells from the original thrombus.
Discussion
Our previous experience with porous metallic stents in the treatment of experimentally created saccular aneurysms was favorable (5). This previous experiment demonstrated stasis of blood within the aneurysmal lumens after deployment of the stent. Blood stasis led to thrombus formation and eventually organization. The saccular aneurysmal lumen was completely obliterated from the intravascular circulation. In the current study, these aneurysms were fusiform, but the typical patterns of stasis with subsequent thrombus formation and organization were again observed. A common element between the two experiments was this stasis of blood and eventual ablation of the residual aneurysmal lumen. The usual mechanical flow characteristics within the saccular aneurysm changed immediately after stent deposition. Usual inflow and outflow effects were not seen after stent placement; instead, gradual puddling of blood and contrast medium was observed immediately after stent deployment. This trapping of blood within the aneurysm led to fibrotic growth and organization and eventual ablation of the aneurysmal pouch (Fig 5). Furthermore, the stent did not cause occlusion of the carotid artery. A thin layer of neointima surrounded the stent wires and incorporated the wires into the wall of the aneurysm. In our model, stents altered blood flow within the aneurysmal lumen by providing a mechanical hindrance to the usual flow patterns while maintaining patency of the native lumen. Histopathologic study revealed mature fibrous connective tissue and collagen, indicating a well-organized thrombus filling each aneurysmal lumen.
fig 5.
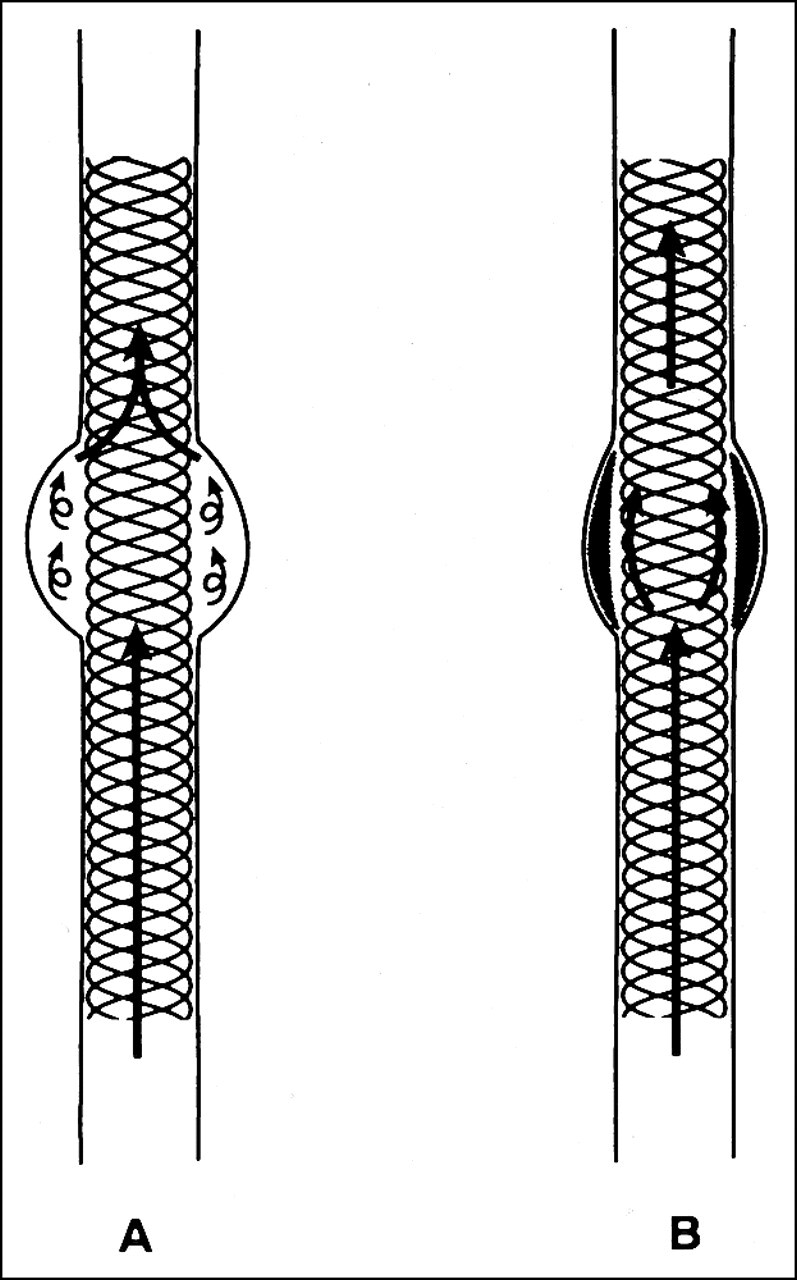
Diagram of flow patterns immediately after stent deployment (A) and 8 weeks later (B). Complex streams of flow are seen within the residual aneurysmal lumen between the outer aneurysmal wall and the stent wires (A). Eight weeks after stent deployment, the aneurysmal lumen is occupied with organized fibrous connective tissue (B)
Wakhloo and coworkers (6, 7) devised an experiment to study the flow patterns within an aneurysm. They used laser-based imaging technology to reveal complex flow patterns within aneurysm models after stent placement across the aneurysmal neck. They demonstrated stagnant flow and reduced flow vortices within the aneurysmal lumen caused by the increased high-flow resistance through the stent wire mesh. This stagnant flow promotes thrombus formation within the aneurysm, as seen in our experiment. Hurst et al (8) reported on the clinical application of stents for treatment of carotid aneurysms. Two cervical internal carotid artery aneurysms were treated with endovascular stents, which were placed across the diseased portion of the aneurysm. After stent placement, angiography revealed immediate reduction in aneurysmal filling. Follow-up angiography at least 6 months later showed occlusion of the aneurysms with preservation of the parent vessels.
In instances in which stents alone are insufficient to alter flow patterns necessary for thrombus formation, stents may serve as a buttress for coils. There are several anecdotal reports of successful treatment of fusiform aneurysms with this combined approach (9–11). Higashida et al (9) placed an intravascular stent within the basilar artery across the base of a fusiform aneurysm with a broad-based neck. Electrically detachable coils were deposited within the dome of the aneurysm. The stent mesh confined the coils to the aneurysmal lumen while preserving flow through the native basilar artery. Mericle et al (11) successfully treated a dissecting pseudoaneurysm of the horizontal segment of the petrous internal carotid artery with a flexible stent and electrically detachable coils. They reported that the stent acted as an “endoluminal scaffold” to prevent coil herniation into the parent artery.
There have been several anecdotal reports of endovascular covered stents for the treatment of arterial aneurysms (12–18). The combination of intravascular stents and prosthetic graft material forms a seal that is relatively impervious to blood. This device is used to treat lesions, such as fusiform aneurysms or fistulas, by excluding them from the circulation. Such stented graft devices can also be used to treat arterial aneurysms and traumatic vascular injuries, such as fistulas. A stent graft is a prosthetic graft fixed to the arterial wall with an attachment device, such as an intravascular stent. Criado et al (13) treated more than 50 patients over a 3-year period with a success rate of greater than 95% using a modified Palmaz stent with a Dacron-knitted graft. These authors describe grafts made of polytetrafluoroethylene that has been sewn onto Palmaz balloon-expandable stents. Examination of pathologic specimens has shown that prosthetic graft incorporation is achieved within the wall of the native artery by establishing a stable flow surface within the lumen of the graft; however, there has been no long-term follow-up with this experience. Nicholson et al (14) reported treating an iatrogenic pseudoaneurysm of the left carotid artery by the insertion of a self-expanding Nitinol stent covered by polyester fabric (Craggstent, Mintec, Bahamas). These stents have been used to treat pseudoaneurysms and aneurysms of the internal iliac arteries. In that study, the pseudoaneurysm was caused by mediastinoscopy. A CT scan revealed a 5-cm pseudoaneurysm, and selective left carotid angiography confirmed the presence of a large pseudoaneurysm arising 3 cm from the left common carotid artery origin. Following surgical exposure, the left carotid artery was punctured and a 9F sheath was passed down at the origin of the left common carotid artery. An 8 × 6-mm Craggstent was placed across the origin of the pseudoaneurysm and released. An 8-mm × 4-cm angioplasty balloon was inflated to ensure full expansion of the stent. The Craggstent is covered, so intimal hyperplasia within the stent should not be a problem. But because of the possibility of intimal growth at the ends of the stent, these authors recommended that the patient be monitored regularly by duplex sonography. Any alteration in velocity or waveform would be an indication for angiography and possibly angioplasty. The authors concluded that the polyester-covered Nitinol stent may play a major therapeutic role in the treatment of pseudoaneurysms.
Ruebben et al (15) treated a skull base aneurysm with a covered stent. Angiography revealed an aneurysm of the cervical part of the left internal carotid artery before it enters the skull. It had a maximum diameter of 2 cm, and was partly occupied by thrombus. The aneurysm was excluded with a self-expandable stent of Nitinol (Cragg Endo Pro System I, Mintec) covered with polyester and coated with low-molecular-weight heparin. This device was chosen because of the relatively small diameter of the 5-mm internal carotid artery. The Cragg Endo Pro System I allows an introduction kit of 7F. Reubben et al (15) did this procedure with general anesthesia and exposure of the internal carotid artery. Once the stent was deployed, they dilated the expandable stent with an angioplasty balloon to obtain full expansion. Angiography after stent placement showed the aneurysm's appearance. A 3-month follow-up study showed exclusion of the aneurysm and patency of the artery. These authors concluded that endoluminal stent grafting is a viable method to repair vessels. Use of this new, minimally invasive technique with a high internal carotid artery aneurysm avoided a long operation involving removal of the mandible. It is still uncertain whether placement of an uncovered stent can prevent rupture of internal carotid artery aneurysms, because thrombosis alone might not prevent rupture of an aneurysm, as aortic aneurysms have shown.
Unlike covered stent grafts, porous stents do not cause acute exclusion of an aneurysmal lumen from the native vessel lumen. In our experiment, blood flowed through the stent's wire mesh into the aneurysmal lumen immediately after stent placement. Flow patterns within the aneurysm, however, were altered to meet conditions favorable for thrombus formation and organization. Consequently, the aneurysmal lumens were eventually excluded from the native circulation. Ideally, covered stents would be used to exclude fusiform aneurysms acutely; however, recent anecdotal reports have revealed intimal hyperplasia at the proximal and distal ends of the covered stents, which could result in stenosis or potential occlusion of the native vessel. This intimal hyperplasia has not been seen at the ends of porous stents. Porous stents may offer eventual exclusion of fusiform aneurysms without the stenotic effects of covered stents.
Intravascular stents have been widely used clinically to maintain patency in vessels stenosed by atherosclerotic disease (19). In the laboratory, experiments have revealed that intravascular stents may have promise in treating certain vascular lesions (20, 21). The stent wires were covered by a fine neointima except those sites in which the wire crossed a communicating channel. Stent wires not covered by neointima may act as a potential nidus for platelet aggregation, as was seen in our experiment. For this reason, the use of stents to treat fusiform aneurysms in humans needs further investigation.
Commercially available stents have not been engineered for intracranial applications. The majority of available stents are too rigid to negotiate the inherent tortuous vascularity of the intracranial circulation. For this reason, there are few cases of successful stent deployment within these vessels. More malleable and lower profile stents will be necessary before their application in the treatment of intracranial vascular disease could become routine.
Another theoretical pitfall in the use of stents would be the potential to occlude vessels extending off the stented native vessel caused by neointimal proliferation. This could have grave consequences in small vessels, such as the basilar artery (eg, brain stem stroke). An experiment in canine models documented neointimal proliferation extending into the origins of branching vessels in response to a stent (22). No vessel, however, was occluded or radiographically stenosed, but the caliber of these arteries, however, was significantly larger than the small perforators originating from the basilar artery. Further experimental and clinical experience with stents in the small vessels with perforating branches will be necessary before the true risk to patients considered for this therapy can be ascertained.
Conclusion
The purpose of this study was to demonstrate the effectiveness of endovascular stents in the treatment of experimental fusiform aneurysms. The wire mesh of the porous stent interfered with the usual blood flow patterns within the aneurysmal lumen, which resulted in fibrosis and contraction of the aneurysm's outer wall. Patients with carotid fusiform aneurysms initially present with neurologic deficits caused by nerve compression from the expanded diameter of the carotid artery lumen. Contraction of the outer walls of the aneurysm around a stent, as seen in our experiment, may reverse these symptoms. Furthermore, this experiment demonstrated that porous stents effectively exclude the aneurysm from the normal circulation while preserving the lumen of the native carotid artery. Thus, stenting could eliminate the morbidity and mortality associated with carotid occlusion, the only available endovascular or surgical therapy for carotid fusiform aneurysms.
Footnotes
Address reprint requests to Glen Geremia, MD, Dept of Radiology, Rush-Presbyterian-St Luke's Medical Center, 1653 W Congress Parkway, Chicago, IL 60612.
References
- 1.Gonzalez CF, Moret J. Balloon occlusion of the carotid artery prior to surgery for neck tumors. AJNR Am J Neuroradiol 1990;11:649-652 [PMC free article] [PubMed] [Google Scholar]
- 2.Lasjaunias P, Berenstein A. Surgical Neuroangiography: 2 Endovascular Treatment of Craniofacial Lesions.. New York: Springer; 1987:248-260 [Google Scholar]
- 3.Palmaz J, Laborde J, Rivera F, Encarnacion C, Lutz J, Moss J. Stenting of the iliac arteries with the Palmaz stent: experience from a multicenter trial. Cardiovasc Intervent Radiol 1992;15:291-297 [DOI] [PubMed] [Google Scholar]
- 4.Strecker E, Liermann D, Barth K, et al. Expandable tubular stents for treatment of arterial occlusive diseases: experimental and clinical results. Radiology 1990;175:97-102 [DOI] [PubMed] [Google Scholar]
- 5.Geremia G, Haklin M, Brennecke L. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol 1994;15:1223-1231 [PMC free article] [PubMed] [Google Scholar]
- 6.Wakhloo A, Lieber B, Stancampiano A, Hopkins L. Effect of intravascular stents on flow characteristics in an aneurysm model. Circulation 1996;94(Suppl):1-598964107 [Google Scholar]
- 7.Wakhloo A, Lanzino G, Lieber B, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery 1998;43:377-379 [DOI] [PubMed] [Google Scholar]
- 8.Hurst R, Haskal Z, Zager E. Endovascular stent treatment of cervical internal carotid artery aneurysms with parent vessel preservation. Surg Neurol 1998;50:313-317 [DOI] [PubMed] [Google Scholar]
- 9.Higashida R, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 10.Perez-Cruet M, Patwardhan R, Mawad M, et al. Treatment of dissecting pseudoaneurysm of the cervical internal carotid artery using a Wallstent and detachable coils: case report. Neurosurgery 1997;40:622-625 [DOI] [PubMed] [Google Scholar]
- 11.Mericle R, Lanzino G, Wakhloo A, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery 1998;43:1229-1234 [DOI] [PubMed] [Google Scholar]
- 12.Marin M, Veith F. Endovascular stents and stented grafts for the treatment of aneurysms and other arterial lesions. Adv Surg 1996;29:93-109 [PubMed] [Google Scholar]
- 13.Criado E, Marston W, Ligush J, Mauro M, Keagy B. Endovascular repair of peripheral aneurysms, pseudoaneurysms and arteriovenous fistulas. Ann Vasc Surg 1997;11:256-263 [DOI] [PubMed] [Google Scholar]
- 14.Nicholson A, Cook A, Dyet J, Galloway J. Case report: treatment of a carotid artery pseudoaneurysm with a polyester covered nitinol stent. Clin Radiol 1995;50:872-875 [DOI] [PubMed] [Google Scholar]
- 15.Ruebben A, Merlo M, Verri A, et al. Exclusion of an internal carotid aneurysm by a covered stent. J Cardiovasc Surg 1997;38:301-303 [PubMed] [Google Scholar]
- 16.Dorffner R, Winkelbauer F, Kettenbach J, Staudacher M, Lammer J. Successful exclusion of a large femoropopliteal aneurysm with a covered nitinol stent. Cardiovasc Intervent Radiol 1996;19:117-119 [DOI] [PubMed] [Google Scholar]
- 17.Krajcer Z, Khoshnevis R, Leachman D, Herman H. Endoluminal exclusion of an iliac artery aneurysm by Wallstent endoprosthesis and PTFE vascular graft. Tex Heart Instit J 1997;24:11-14 [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseau H, Gieskes L, Joffre F, et al. Percutaneous treatment of peripheral aneurysms with the Cragg Endopro System. J Vasc Intervent Radiol 1996;7:35-39 [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum G, Lefkowitz M, Giannotta S. Carotid angioplasty and stenting in high-risk patients. Surg Neurol 1998;50:300-311 [DOI] [PubMed] [Google Scholar]
- 20.Geremia G, Bakon M, Brennecke L, Haklin M. Experimental arteriovenous fistulas: treatment with porous metallic stents. AJNR Am J Neuroradiol 1995;16:1965-1973 [PMC free article] [PubMed] [Google Scholar]
- 21.Geremia G, Bakon M, Brennecke L, Haklin M, Silver B. Experimental arteriovenous fistulas: treatment with silicone-covered metallic stents. AJNR Am J Neuroradiol 1997;18:271-277 [PMC free article] [PubMed] [Google Scholar]
- 22.Geremia G, Kim T, Haklin M, Brennecke L, Douglas J. Intravascular stents: effects on branching vessels originating from a stent containing parent artery. Presented at the annual meeting of the American Society of Neuroradiology, St Louis, May 1992