Abstract
Summary: We present the MR imaging, CT, and clinical findings of a patient with malignant schwannoma of the trigeminal nerve. Local tumor recurrence is frequent and may be mistaken for lymphatic spread. In this report, we emphasize the natural history of this rare tumor and discuss the importance of imaging in diagnosis and surveillance.
Schwannomas of the cranial nerves are usually benign, involving the vestibular nerve and, less often, the trigeminal nerve. Malignant schwannomas of the cranial nerves are much less common. Unlike other malignant schwannomas, those involving the trigeminal nerve are not associated with von Recklinghausen disease and seem to arise de novo or, rarely, through malignant transformation of preexisting benign schwannomas (1). Distal perineural extension of this tumor is an important mechanism of tumor recurrence and should not be mistaken for lymphadenopathy. We present an unusual case of malignant schwannoma of the trigeminal nerve that recurred 3 years after radiation therapy as perineural spread to the face.
Case Report
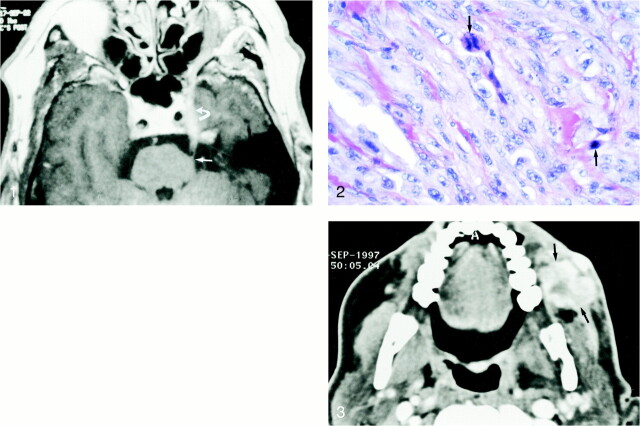
A 71-year-old man was examined because of facial pain in the distribution of the second division of the trigeminal nerve. The intensity of the facial pain progressed over several months and extended to involve the third division of the trigeminal nerve. The patient also complained of facial numbness, and weakness of the muscles of mastication was noted during physical examination. MR imaging showed abnormal thickening and enhancement of the trigeminal nerve, particularly involving the second division (Fig 1). The third division of the trigeminal nerve at the level of Meckel's cave also appeared abnormal. The irregular margins of the lesion and extent of spread prompted a biopsy for diagnosis.
fig 2.
fig 1. Unenhanced axial T1-weighted MR image (728/15/2 [TR/TE/excitations]; matrix, 256 × 512; section thickness, 4 mm) shows an abnormally thickened second division of the trigeminal nerve involving the nerve root entry zone (short arrow) and extending through the prepontine and cavernous (curved arrow) segments.Photomicrograph (hematoxylin and eosin stain) of the original biopsy specimen shows intertwined spindle-shaped cells with pleomorphic hyperchromatic nuclei and frequent mitotic figures (arrows).fig 3. Axial contrast-enhanced CT section obtained through the level of the mandible shows heterogeneously enhancing nodular soft tissue (arrows) within the buccal space. Perineural infiltration along peripheral nerve fibers was confirmed by biopsy.
A biopsy of the infraorbital nerve (V2) showed infiltrating tumor composed of loosely woven spindle cells having enlarged hyperchromatic pleomorphic nuclei and frequent mitotic figures (Fig 2). Immunohistochemical stains and electron microscopy confirmed a Schwann cell origin of this malignant tumor. The tumor was thought to be unresectable because of the extent of spread, and 3D conformal radiation therapy was therefore performed for local control and pain relief. A dose of 1800 cGy was administered using external beam radiation, and an additional 6000 cGy was directed at the tumor by use of stereotactic radiosurgery.
The patient initially complained of transient hearing loss, residual facial pain, and paresthesia of the left side of the face. These symptoms resolved during the next 3 months, and follow-up MR imaging showed a decrease in the size of the tumor. The patient's symptoms and MR imaging results remained stable for 3 years, at which time he noticed swelling of the left side of the face. A physical examination revealed several firm, mobile, subcutaneous nodules of the left cheek. CT of the face and neck (Fig 3) showed several soft-tissue nodules in the left infraorbital and buccal regions, which were thought to be lymphadenopathy. Biopsy, however, showed a malignant schwannoma extending along the peripheral nerves of the face. No further therapy was undertaken.
Discussion
Benign schwannoma of the trigeminal nerve comprises only 0.2% to 0.4% of all intracranial tumors and primarily arises in the gasserian ganglion (1). Malignant schwannoma of the trigeminal nerve is even more rare. Malignant schwannomas of peripheral and cranial nerves often are associated with neurofibromatosis type 1. This association, however, has not been shown with malignant schwannoma of the trigeminal nerve. The true incidence of malignant schwannoma of the trigeminal nerve is difficult to determine because of its sporadic nature and the many terms previously used to describe this lesion, including malignant neurofibroma, neurofibrosarcoma, neurosarcoma, neurogenic sarcoma, malignant neurilemmoma, and malignant nerve sheath tumor (1, 2).
Tumors of the trigeminal nerve most often clinically present with facial pain, as was the case with our patient (3). This pain is usually described as burning in nature. Sensory paresthesias and a diminished corneal reflex also may be seen. Motor dysfunction of the muscles of mastication occurs late as the tumor enlarges to involve the third division of the trigeminal nerve. Growth within the cavernous sinus may further lead to dysfunction of cranial nerves III, IV, and VI, and enlargement within the prepontine cistern may lead to compressive effects on cranial nerves VII, VIII, and IX (3).
The imaging features of a malignant schwannoma of the trigeminal nerve may be difficult to distinguish from those of a benign trigeminal nerve schwannoma. The diagnosis may be suggested on the basis of rapid growth or extensive nerve involvement, as observed in our case, with extension from the nerve root entry zone to the level of the infraorbital foramen. Unlike benign cranial nerve schwannomas, malignant lesions tend to cause earlier erosion of the basilar foramina of the skull that may appear out of proportion to the size of the schwannoma (3, 4). Confirmation of malignant schwannoma of the trigeminal nerve, however, generally requires tissue diagnosis.
There are several features revealed by light microscopy that are suggestive of the correct diagnosis. Cellular characteristics of malignant schwannoma consist of a mixture of plump spindle and polyhedral cells with large hyperchromatic pleomorphic nuclei (3). Increased mitotic figures, necrosis, perineural extension along nerve trunks, and epineural invasion with occasional invasion of adjacent structures help confirm the diagnosis (1, 5, 6). Immunohistochemical markers, including S-100 protein, leucine 7, and myelin basic protein, help separate malignant schwannoma from other spindle cell–type tumors (1). S-100 protein was strongly positive in our case and is positive in approximately 50% to 70% of cases of malignant schwannomas, helping to differentiate it from other typical S-100 protein-negative tumors, such as malignant fibrous histiocytoma, fibrosarcoma, and synovial cell sarcoma (4). Further analysis of the S-100 α and β subunits may be helpful; there is evidence suggesting reverse expression in malignant schwannoma (positive S-100α subunit, negative S-100β subunit) as compared with normal Schwann cells (negative S-100α subunit, positive S-100β subunit) (1, 7). Electron microscopy was not performed in our case, but findings such as interdigitating cytoplasmic processes, scanty intercellular junctions, focal basal lamina, and interstitial collagen fibers help confirm Schwann cell origin (1).
Considering the rarity of malignant schwannoma of the trigeminal nerve, appropriate management of these patients remains unclear. Complete surgical resection is often not possible because of the location and extensive nature of the lesion that is often present at the time of diagnosis. Lymphatic spread of malignant schwannoma is rare; therefore, neck dissection is usually not indicated (5, 6). Hematogenous metastasis generally occurs as a late event with spread to lungs or bone in ≤33% of patients with malignant schwannomas of the head and neck (6, 8, 9). An initial chest radiograph at the time of diagnosis may therefore be useful for future follow-up. Further staging should include CT of the chest and a bone scan as the clinical history or symptoms warrant (9). Radiation therapy has shown varying results but often is the only therapeutic option because of the location of malignant schwannoma of the trigeminal nerve. Chemotherapy seems to have a very limited role in the management of this tumor (10).
Local recurrence by perineural extension may occur proximal or distal to the original tumor and may occur several months to years after initial therapy (5). This was particularly true in the present case in which perineural spread along the facial distribution of the trigeminal nerve fibers was observed 3 years after initial diagnosis and treatment. Nodular metastases of the skin due to perineural spread was also described in a case reported by Karmody (8) and may present a challenge in local control using radiation therapy alone. As many as 50% of malignant schwannomas locally recur despite adjuvant radiotherapy and negative surgical margins (9). Despite best therapeutic efforts, malignant schwannomas are very difficult to cure, with 5-year survival rates reported as being from 37.6% (11) to 65.7% (12). The potential for local recurrence should be considered in planning for appropriate therapy and imaging surveillance and should not be confused with lymphatic spread to regional lymph nodes.
Footnotes
Address reprint requests to Jeffrey A. Stone, MD, Department of Radiology, Medical College of Georgia, Augusta, GA 30912.
References
- 1.Horie Y, Akagi S, Taguchi K, et al. Malignant schwannoma arising in the intracranial trigeminal nerve: a report of an autopsy case and a review of the literature. Acta Pathol Jpn 1990;40:219-225 [DOI] [PubMed] [Google Scholar]
- 2.Maroun FB, Sadler M, Murray GP, et al. Primary malignant tumours of the trigeminal nerve. Can J Neurol Sci 1986;13:146-148 [DOI] [PubMed] [Google Scholar]
- 3.Hedeman LS, Lewinsky BS, Lochridge GK, Trevor R. Primary malignant schwannoma of gasserian ganglion. J Neurosurg 1978;48:279-283 [DOI] [PubMed] [Google Scholar]
- 4.Colmenero C, Rivers T, Patron M, Sierra I, Gamallo C. Maxillofacial malignant peripheral nerve sheath tumours. J Craniomaxillofac Surg 1991;19:40-46 [DOI] [PubMed] [Google Scholar]
- 5.Robertson I, Cook MG, Wilson DF, Henderson DW. Malignant schwannoma of cranial nerves. Pathology 1983;15:421-429 [DOI] [PubMed] [Google Scholar]
- 6.David DJ, Speculand B, Vernon-Roberts B, Sach RP. Malignant schwannoma of the inferior dental nerve. Br J Plast Surg 1978;31:323-333 [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Takahashi K, Sonobe H, Ohtsuki Y, Taguchi K. The distribution of alpha and beta subunits of S-100 protein in malignant schwannomas arising from neurofibromatosis of von Recklinghausen's disease. Virchows Arch A Pathol Anat Histopathol 1987;411:515-521 [DOI] [PubMed] [Google Scholar]
- 8.Karmody CS. Malignant schwannoma of the trigeminal nerve. Otolaryngol Head Neck Surg 1979;87:594-598 [DOI] [PubMed] [Google Scholar]
- 9.Bailet JW, Abemayor E, Andrews JC, Rowland JP, Fu YS, Dawson DE. Malignant nerve sheath tumors of the head and neck: a combined experience from two university hospitals. Laryngoscope 1991;101:1044-1049 [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi M, Tanaka Y, Tutui S, Bann S. Extensive malignant schwannoma of the mandibular nerve: case report. Int J Oral Maxillofac Surg 1992;21:280-281 [DOI] [PubMed] [Google Scholar]
- 11.Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma: clinical characteristics, survival, and response to therapy. Cancer 1981;47:2503-2509 [DOI] [PubMed] [Google Scholar]
- 12.Dewan SK, Bihani VK, Mehta PA. Malignant schwannoma: a clinicopathological study. Cancer 1973;31:184-1904683036 [Google Scholar]