Abstract
BACKGROUND: Previous studies to determine memory lateralization with functional MR imaging (fMRI) have used encoding or recall tasks. The convergence between the results of both tasks, however, is unknown.
OBJECTIVE: The objective of this study was to investigate hemispheric asymmetries of temporal lobe activity (parahippocampus and fusiform gyri) in patients with temporal lesions by using both kinds of fMRI tasks.
METHODS: By using blood oxygenation level–dependent fMRI, hemispheric asymmetries of 25 consecutive patients admitted for presurgical evaluation of memory and 12 healthy control participants were studied. Activation was induced by using the picture-encoding task (processing of complex scenes) and the hometown-walking task (requiring mental navigation through one’s hometown by using landmarks given by participants themselves).
RESULTS: Results in the control group showed that both tasks activated the parahippocampus similarly. The picture-encoding task, however, yielded greater posterior activations in the parahippocampus than did the hometown-walking task. As observed in other studies, more than half the patients showed contralesional representation of memory in each task. It is important to note that estimated memory lateralization from each task was different in 30% of patients, and several cases showed clear discrepancies between both tasks.
CONCLUSION: Although previous studies showed that both tasks were useful for evaluating memory lateralization, the present study suggested that the administration of both tasks is necessary for presurgical evaluation of memory lateralization in patients with lesions in the temporal lobe. Therefore, both encoding and recall processes should at least be considered in the evaluation of memory.
The assessment of mesial temporal lobe (MTL) function is important in patients with brain tumors in the temporal lobe or patients with medically refractory seizures. There is longstanding evidence from animal and clinical lesional studies that declarative memory depends on the integrity of relevant structures of the temporal lobe such as the hippocampus, the parahippocampus, and the fusiform gyrus.1 Presurgical assessment of declarative memory in patients with temporal lesions is essential to evaluate the incidence of treatment on cognitive functioning. Lesion studies indicate that left and right MTL structures are essential for verbal and visuospatial memory, respectively.2–5 In recent years, there has been an increasing interest in the utility of functional MR imaging (fMRI) for presurgical evaluation of memory. This is because the Wada test has some problems in evaluating memory functions and its capacity to predict postsurgical memory impairment is reduced6,7
There is no established fMRI protocol to evaluate memory functions presurgically. The evaluation of memory is problematic because it is sometimes necessary to infer the lack of participation of ipsilesional temporal lobe in memory functions from a lack of activation.7–13 A predominantly contralesional activation in these structures in patients with unilateral brain damage in the temporal lobe is typically interpreted as a lack of involvement of ipsilesional lobe in memory functions. This conclusion, however, may be misleading in some cases because the lack of activation observed with fMRI may be attributed to other causes, such as processing strategy.10
A few fMRI studies have been conducted to evaluate memory in an individual patient. Perhaps the most used task is the picture-encoding task,8,9,11 which is based on the implicit or explicit codification of complex pictures. This task typically activates bilaterally fusiform and parahippocampal gyrus (but not the hippocampus). In contrast, patients show contralesional or bilateral activations concordant with results of the Wada test. The concordance of both methods is 76% across 3 studies.8,9,11 Further, ipsilesional activation in the picture-encoding task correlated negatively with memory decline after surgery.12 A different study proposed a recall task based on individually familiar visuospatial knowledge7,10,13 In Roland’s hometown-walking test,14 participants were required to mentally navigate a walk through their hometowns, by using some landmarks that they themselves had provided at the outset. This task was shown to reliably activate bilaterally and symmetrically the MTL structures in healthy subjects. In candidates for unilateral temporal resection, the activation was predominantly contralesional in 75% of cases with MTL epilepsy mainly because of hippocampal sclerosis or tumors.13
The MTL structures are involved in both encoding and retrieval processes.5,15–17 One of the advantages of fMRI over the Wada test is that several paradigms may be administered to the same patient because it is a noninvasive procedure. Hence, the debate about whether memory should be assessed by using codification or recall6 may be resolved by studying both. By using fMRI in healthy subjects and patients with unilateral lesions in the temporal lobe, the current study investigates the utility of the picture encoding task and Jokeit’s mental navigation task in determining hemispheric asymmetries in MTL activation. Our working hypothesis was that both paradigms would be concordant for all the cases.
Methods
Participants
We studied 12 healthy control participants (mean age, 43.33 years; age range, 26–62 years) and 25 consecutive patients (mean age, 44.68 years; age range, 23–72 years) with brain lesions affecting the temporal lobe and admitted for fMRI presurgical evaluation of memory. Fifteen had lesions on the left and 10 on the right temporal lobe. Table 1 includes the main characteristics of patient and control participants. Participants gave their informed consent before starting the study. Five patients did not complete the picture-encoding task because of technical problems with the goggles.
Table 1:
Patient characteristics and results from fMRI tasks
| Patient No./Ae (y)/Sex | Temporal Lobe | Etiology | Hippocampal Formation Affected | TLE | L1 Hometown Walking Task | L1 Picture Encoding Task |
|---|---|---|---|---|---|---|
| 1/30/M | L | Glioblastoma multiforme grade IV | Yes | No | −93.35 | −40.90 |
| 2/28/M | L | Cavernous angioma | Yes | No | 9.30 | 8.20 |
| 3/44/F | L | Glioblastoma multiforme grade IV | Yes | Yes | −83.57 | −35.13 |
| 4/61/M | R | Glioblastoma multiforme grade IV | Yes | No | 61.77 | 98.37 |
| 5/61/M | R | Cavernous angioma | Yes | Yes | 18.31 | 30.69 |
| 6/39/M | R | Glioblastoma multiforme grade IV | Yes | No | 29.17 | 31.99 |
| 7/51/F | L | Cavernous angioma | No | Yes | −36.20 | −29.57 |
| 8/36/M | R | Low-grade astrocytoma | Yes | No | 16.83 | |
| 9/70/M | L | Glioblastoma multiforme grade IV | Yes | Yes | −43.71 | −75.25 |
| 10/64/M | L | AVM | Yes | No | 11.82 | |
| 11/30/F | L | Oligoastrocytoma grade II | Yes | Yes | −100 | |
| 12/39/F | R | Hippocampal sclerosis | Yes | Yes | 17.05 | −2.14 |
| 13/72/M | L | High-grade glioma | No | No | −36.35 | −85.52 |
| 14/43/M | L | AVM | Yes | Yes | −23.66 | |
| 15/55/F | R | Oligodendroglioma grade II | Yes | Yes | 57.54 | 48.55 |
| 16/34/M | R | Low-grade astrocytoma | Yes | Yes | 19.56 | 6.18 |
| 17/27/F | L | Meningothelial meningioma | No | No | −9.21 | −99.00 |
| 18/33/F | R | Cavernoma | No | Yes | 8.85 | −29.61 |
| 19/47/M | L | Glioblastoma multiforme grade IV | Yes | Yes | 53.27 | −35.66 |
| 20/42/F | L | Cavernous angioma | Yes | Yes | −64.09 | 1.26 |
| 21/26/F | L | Medial temporal lobe atrophy | Yes | Yes | −8.67 | −8.68 |
| 22/37/F | L | AVM | Yes | No | −100 | −100 |
| 23/50/M | L | Tumoral lesion* | Yes | No | −40.29 | −25.44 |
| 24/46/F | R | Hippocampal sclerosis | Yes | Yes | 54.92 | |
| 25/23/M | R | Low-grade glioma | Yes | Yes | 40.09 | 8.79 |
| Controls | ||||||
| 1/26/F | 17.86 | 9.01 | ||||
| 2/60/F | −7.28 | 3.23 | ||||
| 3/62/F | −4.01 | 11.66 | ||||
| 4/26/F | 6.51 | −14.00 | ||||
| 5/28/F | 5.60 | −7.07 | ||||
| 6/45/M | −5.70 | −13.23 | ||||
| 7/40/F | −5.71 | 0.47 | ||||
| 8/47/F | −10.02 | 18.37 | ||||
| 9/39/F | 0.24 | 3.02 | ||||
| 10/59/M | 7.94 | −7.87 | ||||
| 11/43/F | −14.96 | −6.35 | ||||
| 12/45/F | −12.93 | 17.99 |
Note:—TLE indicates temporal lobe epilepsy; LI, lateralization index; AVM, arteriovenous malformation.
No histological diagnosis.
Memory Evaluation
Seventeen patients (11 with lesions on left and 6 on the right temporal lobe) underwent a comprehensive neuropsychological test battery. For correlative purposes, we selected the following tests: (1) Rey complex figure delayed free recall—recall performance includes number of correctly remembered details and correct spatial positions; (2) digit span test from the Wechsler Adult Intelligence Scale (WAIS-III) (standard score); and (3) Rey auditory verbal learning test (RAVLT)—the number of correctly recalled words in the first and fifth trial and the number of words recalled after 30 minutes.
fMRI Tasks
Participants completed consecutively in the same order 2 different memory paradigms of 6 minutes, with 6 activation and 6 baseline blocks. The duration of each block was 30 seconds. The first paradigm was the picture-encoding task.8 The activation condition presented 10 different photographs of scenes for 2.5 seconds, followed by 0.5 seconds of a black screen. Sixty different pictures were presented across 2 imaging runs. The pictures were directly obtained from outdoor scenes of different locations in and around their town and also from the Internet. The baseline condition consisted of repeatedly viewing a single nonsense pixelated image. As in the activation condition, each image was also presented for 2.5 seconds, with 0.5 seconds of a blank screen between presentations. Before the scan, participants were instructed to memorize the photographs and just look at the control image. They were also informed that their performance would be later tested outside the scanner. They also did some practice trials for 2 minutes. The stimuli were presented by using Presentation software (Neurobehavioral Systems, Inc., Albany, Calif) and projected by using VisuaStim XGA goggles (Resonance Technology, Northridge, Calif) with a resolution of 1024 × 768 pixels.
After the scans, participants were tested to establish the efficiency of the encoding of visual information. After a previous study,8 they were presented with a sequence of 32 pictures consisting of 8 targets and 24 distractors and were asked to decide whether they had previously seen each photograph during the scanning session; participants responded verbally and the experimenter recorded their answers. Recognition scores (RS) were calculated by applying the following formula: RS = [Hits − (False Alarms/3)].
The second task used to evaluate memory was an adaptation of Roland’s hometown-walking task.10 Each block was introduced by spoken commands by using built-in devices. The task was explained to the participants in detail before scanning. On the basis of the information given by each subject, an individual hometown walk encompassing 10 destinations was prepared. If a participant had recently moved, the most familiar hometown was chosen. We then prepared 6 different walks based on 3 different landmarks plus 2 more for practice before the scanning. In each case, the task started giving 3 landmarks as a basis for the walk, and participants were asked to mentally navigate through the route and to imagine as many details as possible while navigating. Participants were also instructed not to try to get to the end of the route quickly, but if this occurred, they could walk to another point of interest or choose a location from their last destination and imagine what they would see from that point. The baseline task consisted of covertly counting odd numbers starting with 21. Feedback about performance was requested once finished.
MR Imaging Acquisition
Participants were scanned in a General Electric SIGNA CVI 1.5T high-speed imaging device (GE Medical Systems, Milwaukee, Wis) by using a quadrature head coil. Initially, a sagittal localizer scan was run to provide a reference for section selection in later scans. Next, shimming was carried out to maximize field homogeneity. Functional images were collected by using a gradient-echo sequence sensitive to blood oxygenation level-dependence (BOLD). The sequence parameters used were as follows: retention time (TR) = 3000 milliseconds; echo time (TE) = 60 milliseconds; flip angle = 90°. During the fMRI session, 10 sets of 12 oblique coronal sections (section thickness = 5 mm; gap = 2 mm; field of view = 24 cm; matrix size = 128 × 128) were acquired perpendicular to the hippocampus in each block. These parameters allowed for imaging of the entire temporal and parietal lobes, whereas we covered partially occipital and frontal lobes. At the beginning of each MR imaging session, anatomic high-resolution reference images were acquired as a set of 124 contiguous sagittal sections by using a T1-weighted 3D fast-spoiled gradient (FSPGR) sequence with the following parameters: TR = 11 milliseconds; TE = 4.2 milliseconds; section thickness = 1.2; field of view = 24 cm; matrix size = 256 × 256.
fMRI Data Analysis
The images were analyzed by using Brain Voyager software, version 4.9.6 (Brain Innovation, Inc., University of Maastricht, Maastricht, the Netherlands). A different analysis was run with patients and controls. Images obtained in patients were initially corrected by movement, coregistered, and then smoothed with a 6-mm Gaussian filter. The statistical analysis was based on the comparison between activation and baseline tasks in a block design, after removing the first 2 volumes at the beginning of each condition to avoid T1 effect. The same analyses were run for control participants, but images were also transformed to Talairach standard coordinates18 after correction by movement and coregistration. Results of control participants were analyzed as a whole group applying the general linear model. Data were analyzed by using the random effect analysis model (P < .0001).
Voxel-by-voxel t tests were performed for each participant, identifying average signal intensity increases as measured during activation tasks compared with average signal intensity acquired during control conditions. The number of voxels with significant activation (P = .001, uncorrected) were counted in the right and left temporal lobes, including the hippocampus, parahippocampus, and fusiform gyrus12 and expressed as lateralization index (LI): LI = 100 × (L − R)/(L + R), where R and L indicate the number of voxels in the right and left hemispheres, respectively. This approach yields LI ranging between +100 (strong left-hemisphere dominance) and −100 (strong right-hemisphere dominance). LI was classified as indicating a left hemisphere memory dominance when LI >2 SD (obtained from the control group), a bilateral control of memory when LI was between −2 SD and +2 SD, or right hemisphere memory dominance when LI was less than −2 SD. Two separate researchers made the counting for each participant, and results were averaged (correlation between both was high, r = 0.97).
Results
All participants were able to perform the fMRI study and gave feedback reporting that they had performed the tasks according to the instructions given. Figure 1A shows activations during the picture-encoding task and the hometown-walking task for the control group. Consistent with previous reports, both tasks showed strong bilateral activations in the temporal lobe, with emphasis on the parahippocampal gyrus (Fig 1A). Detailed activations for the control group appear in Table 2. The main difference between both tasks was that the picture-encoding task (but not the hometown-walking task) yielded significant activations in fusiform and lingual gyri. A more subtle difference is that the hometown-walking task yielded stronger activations in the anterior portion of left parahippocampal gyrus (Broadmann areas 27 and 35).
Fig 1.
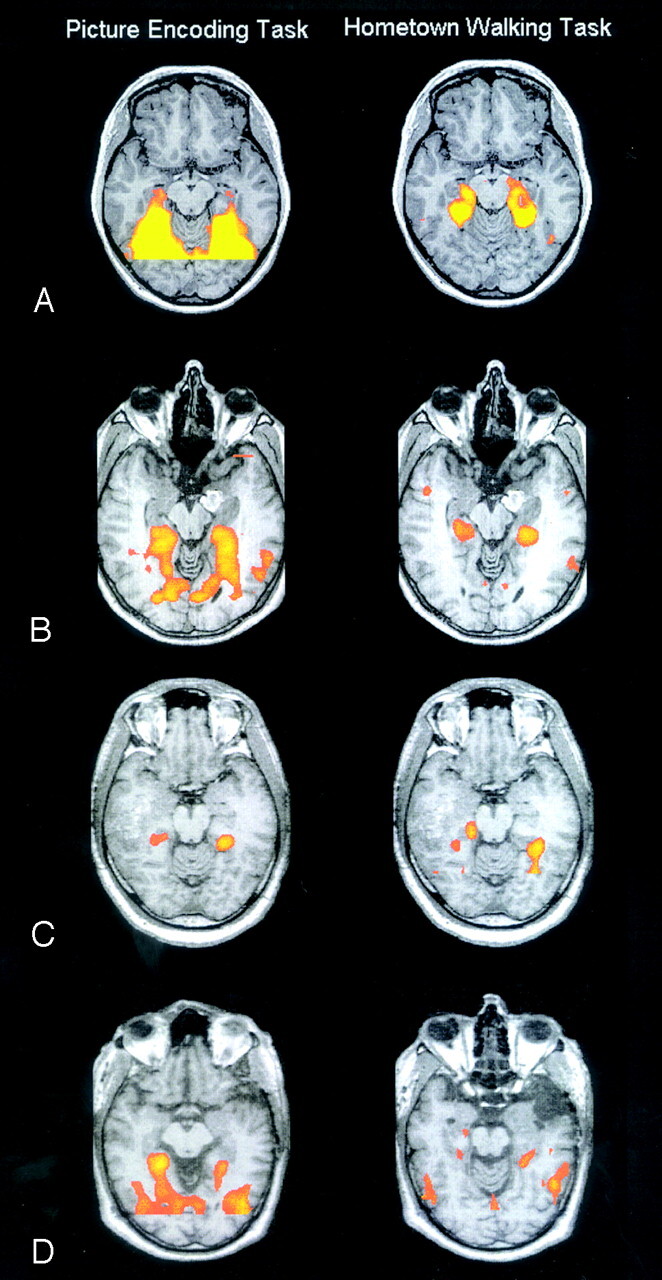
Functional activation results for the picture-encoding task (left) and the hometown-walking task (right). All images are in radiologic orientation. Activation thresholds were at 0.00001 for groups and 0.001 for individual patients.
A, Results for the control group.
B, Results for patient 2 showing symmetric bilateral activation of the temporal lobe for both tasks.
C, Results for patient 6 showing asymmetric activation of the temporal lobe for both tasks.
D, Results for patient 19 showing a clear discordance between the results of both tasks, where the picture-encoding tasks showed a contralesional representation of memory, the hometown-walking task showed an ipsilesional representation of memory.
Table 2:
Brain areas activated during fMRI tasks in the control group
| Brain area | Picture-Encoding Task |
Hometown-Walking Task |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BA | Talairach Coordinate |
Mean t Value | BA | Talairach Coordinate |
Mean t Value | |||||
| x | y | z | x | y | z | |||||
| Supplementary motor area | 6 | 6 | 9 | 50 | 4.80 | 6 | 9 | 12 | 48 | 5.28 |
| Lateral frontal gyrus (R) | 6 | 36 | 10 | 30 | 5.02 | 6 | 24 | −1 | 55 | 4.95 |
| Lateral frontal gyrus (L) | 6 | −26 | −4 | 45 | 4.85 | 6 | −27 | −10 | 54 | 5.32 |
| Parahippocampal gyrus (R) | 19, 27, 28, 30, 35, 36 | 25 | −37 | −5 | 5.28 | 19, 27, 28, 30, 35, 36, 37 | 22 | −29 | −8 | 5.45 |
| Parahippocampal gyrus (L) | 19, 28, 30, 36, 37 | −22 | −42 | −5 | 5.75 | 19, 27, 28, 30, 35, 36, 37 | −20 | −34 | −7 | 5.41 |
| Lingual gyrus (R) | 18, 19 | 14 | −57 | 0 | 6.02 | |||||
| Lingual gyrus (L) | 18,19 | −11 | −56 | 1 | 5.56 | |||||
| Fusiform gyrus (R) | 19, 20, 37 | 33 | −50 | −10 | 6.06 | |||||
| Fusiform gyrus (L) | 19, 20, 37 | −31 | −49 | −11 | 6.10 | |||||
| Posterior cingulate (R) | 23, 29, 30, 31 | 5 | −52 | 8 | 5.62 | 23, 29, 30, 31 | 9 | −49 | 11 | 6.09 |
| Posterior cingulate (L) | 23, 29, 30, 31 | −5 | −52 | 8 | 5.13 | 23, 29, 30, 31 | −6 | −49 | 9 | 6.29 |
| Hippocampus (R) | 27 | −16 | −13 | 4.41 | 27 | −24 | −9 | 4.52 | ||
| Hippocampus (L) | −27 | −16 | −13 | 5.44 | −26 | −24 | −6 | 4.60 | ||
| Thalamus (R) | 5 | −23 | 3 | 8.90 | 11 | −24 | 14 | 5.18 | ||
| Thalamus (L) | −17 | −28 | 2 | 4.98 | −10 | −22 | 10 | 5.48 | ||
Note:—Areas are detailed as a function of gyri and Broadmann’s maps. BA indicates Broadmann areas; L, left hemisphere; R, right hemisphere.
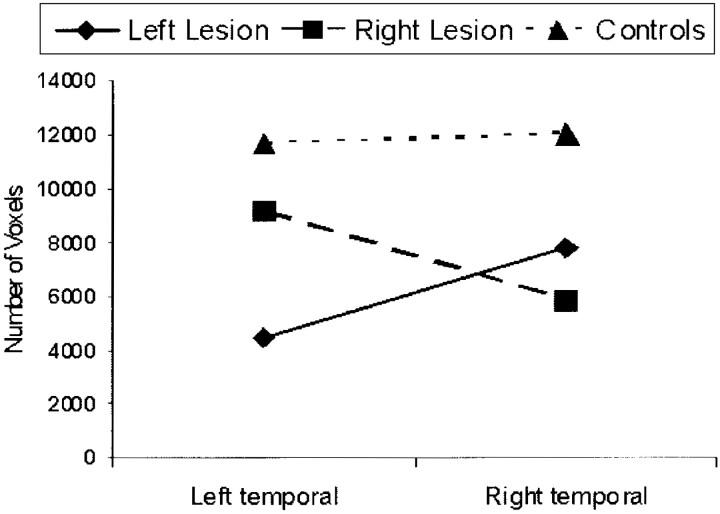
As in previous studies,10,12 group differences were identified through multiple analysis of covariance by using the number of voxels activated as a dependent measure. (The results were very similar if we used LI as dependent variables.) Within-subjects factors were Task (picture encoding vs hometown walking) and Side (left vs right), whereas Group (left lesion, right lesion, and control) was the between-subjects factor. This analysis only yielded a main effect for Task (F 1,26 = 7.93; P < .01), indicating that the picture-encoding task activated more voxels than the hometown-walking task. The only significant interaction was Side × Group (F 2,25 = 8.82; P < .001), showing that patients had a lower number of ipsilesional activated voxels than controls, whereas there was no significant difference between the number of contralesional voxels in patients and controls (Fig 2).
Fig 2.
Mean number of voxels activated for both tasks in each group.
With regard to control subjects, mean LI was −2.73 (SD = 16.47) for the picture-encoding task and −1.87 (SD = 9.72) for the hometown-walking task. Individual data showed no cases with unilateral activations for any task (Table 1). Cutoff scores for determining laterality of representation were obtained by calculating the mean ±2 SD from data of the control group. Then, cutoff scores were −35 and 31 for the picture-encoding task and −22 and 19 for the hometown-walking task.
When patients were analyzed individually, most of them showed contralesional (55% for the picture-encoding task and 64% for the hometown-walking task) or bilateral representations (45% for the picture-encoding task and 32% for the hometown-walking task). Finally, patient 19 showed an ipsilesional dominance of memory by using the hometown-walking task. We conducted qualitative comparisons to study the concordance between information derived from both tasks. The conclusions derived from both tasks were concordant in 70% of patients (45% had contralesional, and 25% bilateral control of memory). This also means, of course, that 30% of patients had a discordant result. Cases such as patients 17, 20, 23, and 25 showed clear discrepancies between the results derived from both tasks, and case 19 obtained the opposite results (LIs, Table 1). Figure 1 also shows examples from different patients.
Spearman rank correlation coefficients between voxel counts in the left and right MTLs, and behavior measures are presented in Table 3. The number of activated voxels within the left MTL was positively correlated with RAVLT scores (a measure of verbal memory), whereas the number of activated voxels within the right MTL (during the picture-encoding task) was positively correlated with delayed reproduction performance of the Rey complex figure recognition score during the fMRI task and digit span. (Because AVM typically produced a decreased activation in perilesional areas, we ran the same correlation analyses after removing patient 22. These correlations did not change the main results of Table 3.)
Table 3:
Correlations between recognition scores on the picture- encoding task and number of left and right voxels activated in both tasks
| Mean (±SD) | Picture-Encoding Task |
Hometown-Walking Task |
|||
|---|---|---|---|---|---|
| Left | Right | Left | Right | ||
| Recognition store | 4.13 (2.46) | .21 | 56* | .44 | .45 |
| RAVLT | |||||
| First trial | 4.71 (1.90) | .65** | .36 | .63** | .14 |
| Five trials | 12.07 (3.64) | .62** | .40 | .67** | .28 |
| Recall (30 min) | 9.36 (4.58) | .57* | .40 | .48 | .20 |
| Rey figure | |||||
| Copy | 64.08 (31.16) | −.02 | .00 | .14 | .30 |
| Recall | 15.77 (13.14) | .17 | .58* | .37 | .38 |
| Digit span | 8.07 (2.3) | .54* | .58* | .22 | .30 |
Note:—
P < .05,
P < .01.
RAVLT indicates Rey Auditory Verbal Learning Task.
Discussion
By use of fMRI tasks, this study has confirmed previous results showing that the picture-encoding and hometown-walking tasks reliably activated MTL structures in control subjects and patients with unilateral lesions in the temporal lobe.7–13 The main objective of this study was to show the concordance between the 2 fMRI tasks. Although both tasks have been used to study memory lateralization, there are important differences between them. The first difference arises from the cognitive processes involved: whereas the picture-encoding task involves codification of new material to be remembered, the hometown-walking task is focused on recall of stored information. The results of the present study reveal additional differences between both tasks.
When the brain areas involved in each task in control participants were compared, the results show important differences. Both tasks showed a great overlap in the bilateral activation of parahippocampal gyrus; however, overlapping between both tasks was not absolute (Table 2). The hometown-walking task activated anterior areas of the left parahippocampal gyrus and the hippocampus, whereas the picture-encoding task greatly activated the fusiform and lingual gyri. In addition, the greater complexity of the hometown-walking task produced stronger activations in the posterior cingulate (related to visuospatial memory processes), in the parietal cortex (related to mental navigations and imagination), and the dorsolateral prefrontal cortex (probably reflecting the need for sequencing and planning). In contrast, the picture-encoding task activates both ventral pathways for analyses of visual properties of stimuli.8,9,11 In conclusion, both tasks have replicated activations obtained in previous studies. The most relevant point is that the recall task has yielded more anterior parahippocampal activations than the codification task. These results are consistent with previous reports showing that activations associated with deep encoding were found in parahippocampal and fusiform gyri, with little activation in the hippocampus,15,16,19,20 whereas retrieval of information also activates more anterior structures of hippocampal formation.17,21–23
Bilateral activations within the parahippocampus support the notion that both tasks may be performed by using both visuospatial and verbal strategies.10 One approach to mapping memory function for presurgical patients is to use tasks that reliably bilaterally activate MTL. Because memory is thought to be represented bilaterally, unilateral activations should be interpreted as reflecting abnormal functioning in the contralateral temporal lobe, though other interpretations are possible. Although the overall group has shown a clear symmetric activation, some normal participants showed slight asymmetric activations in the right (control participants 1, 8, and 12 in Table 1) or the left hemisphere (control participants 4 and 11 in Table 1). These results may be interpreted as showing the predominance of a particular strategy in performing the task.
In patients, lateralization of memory processes within the MTL may be influenced by the temporal lesion. In 55% of patients for the picture-encoding task and 64% of patients for the hometown-walking task, a predominantly contralesional representation of memory was found. Bilateral representations were found in 45% and 32% of patients, respectively. It is important to note that one case for the hometown-walking task showed an ipsilesional representation of memory. Cases like this were already obtained in a previous study by using the picture-encoding task and were concordant with results of the Wada test.8 Compared with previous studies, our fMRI results found a greater number of patients with bilateral distributions than previous reports.8,10 This may be a consequence of the sample used in this study, which is based on consecutive patients, some of them with extrahippocampal lesions. This result is consistent with a previous post hoc result showing this kind of lesion to yield more bilateral representations of memory than in patients with hippocampal sclerosis.10
It is important to note that MTL activation during both tasks was related to memory performance in neuropsychological tests. As expected, activation in the left MTL during both tasks was correlated with performance in verbal learning and recall, whereas activation in the right MTL during the picture-encoding task correlated with performance of the Rey complex figure delayed free recall test. Because lesions of left MTL were associated with a decline of verbal learning and recall and lesions of right MTL caused impairment in visual memory,24 these data gave validity to the use of both fMRI tasks as evaluating verbal and visual memory. In light of previous literature in both tasks, we may conclude that there is substantial support for the use of the picture-encoding task and the hometown-walking task as a presurgical evaluation of lateralization of memory functions in the brain because both (1) showed a good degree of concordance with results of the Wada test8–11; (2) both predict reliably postsurgical memory decline7,12; and (3) both are correlated with presurgical memory performance (present data).
One of the most interesting results in this study is the individual comparison of both tasks in the clinical assessment of memory lateralization. Both tasks have shown an acceptable degree of concordance of 70% (taking the LI cutoff score of 20). Several cases, however, have shown clear discrepancies, which may not be attributed to a particular task being more likely to produce bilateral or contralesional distributions (for instance, patients 17 and 20, who have opposite results). These results imply the need to use different tasks to evaluate memory distribution by using fMRI to make a more accurate clinical decision. There are different reasons for doing this. One is that memory is a complex function based on different processes (ie, encoding, recall, recognition) and that varies depending on the material to be encoded or recalled (faces, words, scenes, etc; see Golby et al9).
The second reason is possibly more relevant. Clinicians mapping memory functioning in presurgical patients may have to take into account that they are seeking a lack of activation of ipsilesional temporal lobe structures rather than contralesional activation. In this sense, mapping of memory is different from other fMRI presurgical assessments such as motor or language functions based on the interpretation of activations. No activation of a determinate brain area obtained in fMRI may be interpreted as a lack of participation of this area in a cognitive function. In brain-damaged patients, however, other interpretations such as a magnetic susceptibility artifact caused by the lesion may explain the lack of activation in the ipsilesional temporal lobe. To conclude, therefore, that a medial temporal lobe is not involved in memory functioning, one needs a greater amount of convergent data derived from multiple tests. In this sense, we may conclude that patients 4 and 22 had a contralesional representation of memory and that patients 2 and 21 had a bilateral representation of memory with more confidence than in other cases. Until these procedures become more reliable, however, clinicians may also consider the possibility of using a complementary test (ie, the Wada test or postoperative results) to validate fMRI results. Even though the Wada test has some limitations,6,7 the lack of these complementary tests and the possible effect of the lesions on the fMRI signal intensity are the main limitations of the present study.
Conclusion
Previous research in patients with unilateral lesions in the temporal lobe has revealed that both picture-encoding and hometown-walking tasks were useful to identify representation of episodic memory in the brain. Most of the patients showed a contralesional representation of memory, but some showed bilateral or ipsilesional representations. Our study has confirmed previous results in both paradigms showing a consistent bilateral MTL activation with a good degree of overlap between tasks. Patients with temporal lesions showed a predominant contralesional MTL activation in both tasks. When interpreting results clinically for determination of memory lateralization, however, data have identified a discordance in 30% of cases.
References
- 1.McGaugh JL. Memory a century of consolidation. Science 2000;287:48–51 [DOI] [PubMed] [Google Scholar]
- 2.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 1972;19:421–46 [DOI] [PubMed] [Google Scholar]
- 3.Hermann BP, Wyler AR, Richey ET, et al. Memory function and verbal learning ability in patients with complex partial seizures of temporal lobe origin. Epilepsia 1987;28:547–54 [DOI] [PubMed] [Google Scholar]
- 4.Rausch R. Effects of temporal lobe surgery on behavior. In: Smith D, Trieman DM, Trimble M, eds. Neurobehavioral problems in epilepsy. New York: Raven Press;1991. :279–92 [PubMed]
- 5.Cabeza R, Nyberg L. Imaging cognition. II. An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000;12:1–47 [DOI] [PubMed] [Google Scholar]
- 6.Ojemann JG, Kelley WM. The frontal lobe role in memory: a review of convergent evidence and implications for the Wada memory test. Epilepsy Behav 2002;3:309–15 [DOI] [PubMed] [Google Scholar]
- 7.Janszky J, Jokeit H, Kontopoulou K, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia 2005;46:244–50 [DOI] [PubMed] [Google Scholar]
- 8.Detre JA, Maccotta L, King D, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology 1998;50:926–32 [DOI] [PubMed] [Google Scholar]
- 9.Golby AJ, Poldrack RA, Illes J, et al. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia 2002;43:855–63 [DOI] [PubMed] [Google Scholar]
- 10.Jokeit H, Okujava M, Woermann FG. Memory fMRI lateralizes temporal lobe epilepsy. Neurology 2001;57:1786–93 [DOI] [PubMed] [Google Scholar]
- 11.Szaflarski JP, Holland SK, Schmithorst VJ, et al. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav 2004;5:244–52 [DOI] [PubMed] [Google Scholar]
- 12.Marcie LR, Narayan VM, Kimberg DY, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain 2004;127:2286–98 [DOI] [PubMed] [Google Scholar]
- 13.Janszky J, Ollech I, Jokeit H, et al. Epileptic activity influences the lateralization of mesiotemporal fMRI activity. Neurology 2004;63:1813–17 [DOI] [PubMed] [Google Scholar]
- 14.Roland PE, Eriksson L, Stone-Elander S, et al. Does mental activity change the oxidative metabolism of the brain? J Neurosci 1987;7:2373–89 [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner AD, Shacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998;281:1188–91 [DOI] [PubMed] [Google Scholar]
- 16.Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain 2001;124:399–412 [DOI] [PubMed] [Google Scholar]
- 17.Mandzia JL, Black SE, McAndrews MP, et al. fMRI differences in encoding and retrieval of pictures due to encoding strategy in the elderly. Hum Brain Mapp 2004;21:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: three-dimensional proportional system an approach to medical cerebral imaging. Stuttgart: Thieme;1988
- 19.Bernstein LJ, Beig S, Siegenthaler AL, et al. The effect of encoding strategy on the neural correlates of memory for faces. Neuropsychologia 2002;40:86–98 [DOI] [PubMed] [Google Scholar]
- 20.Grady CL, McIntosh AR, Rajah MN, et al. Neural correlates of the episodic encoding of pictures and words. Proc Natl Acad Sci U S A 1998;95:2703–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eldridge LL, Knowlton BJ, Furmanski CS, et al. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000;3:1149–52 [DOI] [PubMed] [Google Scholar]
- 22.Gabrieli JDE, Brewer JB, Desmond JE, et al. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997;276:264–66 [DOI] [PubMed] [Google Scholar]
- 23.Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J Neurosci 2000;20:7776–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick C, Murrie V, Cook M, et al. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure 1997;6:213–18 [DOI] [PubMed] [Google Scholar]
- 25.Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 2004;55:569–79 [DOI] [PubMed] [Google Scholar]