Abstract
BACKGROUND AND PURPOSE: The physiological mechanism that gives rise to posterior reversible encephalopathy syndrome (PRES) is currently unknown. We sought to better understand the mechanism of the edema formation in PRES and specifically hypothesized that this edema is caused by increased vascular permeability.
METHODS: Eight consecutive patients with PRES who had been studied by using perfusion MR imaging were retrospectively identified. Perfusion images were obtained using a gradient-echo echo planar sequence with contrast enhancement. Measures of apparent diffusion coefficient (ADC), cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time (MTT) and vascular permeability (K2) were calculated in the affected posterior brain areas and normalized to values obtained in unaffected anterior brain. These values were compared with those found in healthy subjects.
RESULTS: Regions of interest within the posterior affected brain indicated a significant increase in ADC values in all but one patient, a significant decrease in CBV and CBF values in all patients with PRES (average 30% of control), variable changes in MTT, and no measurable change in K2.
CONCLUSION: The decrease in both CBV and CBF in PRES supports the theory of autoregulatory vasoconstriction; changes in K2 were not detected.
Posterior reversible encephalopathy syndrome (PRES) has been associated with hypertension, eclampsia, hypercalcemic crises, and neurotoxic or immunosuppressive substances (1, 2). Clinically, patients with PRES have seizures, visual disturbances, altered mental status, and headaches (1–7). The etiology of PRES is unknown; however, hypotheses include a hypertension-induced autoregulatory failure, resulting vasodilation causing increased capillary hydrostatic pressure with subsequent vasogenic edema. Alternately, excessive arteriolar vasoconstriction could result in decreased blood flow, ischemia, and cytotoxic edema (7). Recent studies of cerebral perfusion in PRES have shown decreased cerebral blood volume (CBV) and cerebral blood flow (CBF) with associated edema (2, 8). In most cases, however, the edema is reversible, without evidence of tissue infarction. Because vasoconstriction should decrease the capillary hydrostatic pressure, we hypothesized that edema might be formed through significantly increased capillary permeability.
Methods
Eight consecutive patients with PRES (age range, 12–66 years; seven females and one male) who were studied with perfusion MR imaging were retrospectively identified. The study was performed with approval from our institutional review board under institutional guidelines that allow for retrospective analysis of patient medical records as long as all patient-identifying information is removed. The patients had perfusion MR imaging at the request of referring clinicians because of clinical symptoms of stroke. Studies were performed from 12 hours to 13 days after onset of symptoms. Data from seven normal volunteers (age range, 22–50 years; six female and one male) who had taken part in a different institutional review board–approved project were used as controls. Patients were imaged using conventional, diffusion-weighted, and perfusion-weighted imaging sequences at 1.5T. Perfusion imaging consisted of a gradient-echo echo planar sequence with a TR of 2.0 s, a flip angle of 60°, a TE of 54 ms, a matrix of 128 × 128, and an FOV of 230 mm repeated 40 times. A single dose of gadolinium-DTPA was injected intravenously at the end of the fifth image acquisition and was followed with saline flush.
Perfusion data were processed off-line in the manner described by Østergaard and Weisskoff to create maps of CBV, CBF, mean transit time (MTT), and permeability constant (K2) (9). The arterial input function used for the singular value decomposition was selected on the basis of voxels adjacent to a middle cerebral artery in each patient.
By using custom software written in Interactive Data Language (RSI Inc., Boulder CO), four regions of interest were drawn in the right and left hemisphere, anterior (unaffected) and posterior (affected) for each subject. These regions of interest were transferred to the corresponding ADC, CBV, CBF, MTT, and K2 maps. Ratios of posterior to anterior values for each variable were calculated for each subject.
K2 is based on the following equation, where ΔR̃ represents the measured change in transverse relaxivity, including T1 contamination effects. ΔR̃ is expressed in terms of the relative weightings of R2 and permeability (K1 and K2, respectively), uncontaminated R2 values ΔR̃2, imaging time (t), and time after first pass of bolus (t′) (9).
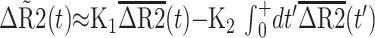 |
1) |
Statistical evaluation.
Comparison of means was by one-way ANOVA with Tukey-Kramer post hoc test by using GraphPad InStat version 3.00 (GraphPad Software, San Diego, CA). Power analysis was performed using Power On X (MMISoftware, Newcastle upon Tyne, United Kingdom).
Results
Of eight subjects with PRES, seven had hypertension; one was receiving chemotherapy for leukemia. Of the subjects with hypertension, two had eclampsia, three had end-stage renal disease, and one was receiving Tacrolimus for imunosupression following lung transplant for cystic fibrosis (Table 1). Conventional MR images of patients with PRES demonstrated typical findings of bilateral cortical and subcortical edema within the parietal and occipital lobes on fluid-attenuated inversion recovery (FLAIR) and T2-weighted images (Figure 1). In seven of the eight patients, ADC values were not decreased in these areas, which indicates that cytotoxic edema was not present (Figure 2). There was no abnormal enhancement evident in any patient.
TABLE 1:
PRES subjects: clinical details and history of patients with PRES and corresponding hemodynamic measures
| Patient | Age/Sex | Time from Onset to Scan | Blood Pressure | Symptoms/Clinical History |
|---|---|---|---|---|
| 1 | 38/F | <1 day | 180/110 | Chronic hypertension, postpartum eclampsia |
| 2 | 66/F | 2 days | 198/83 | Hypertension |
| 3 | 19/M | 4 days | 200/100 | Hypertension, ESRD, altered mental status |
| 4 | 44/F | 1 day | 240/115 | Hypertension and eclampsia |
| 5 | 12/F | 8 days | 129/79 | Hypertension nephrotic syndrome, seizures |
| 6 | 42/F | 13 days | 180/100 | Hypertension, ESRD, hypercalcemia |
| 7 | 29/F | <1 day | 172/91 | Hypertension, cystic fibrosis, pancreatitis |
| 8 | 23/F | 8 days | 104/76 | Pre-B cell ALL |
Note.—F, female; M, male.
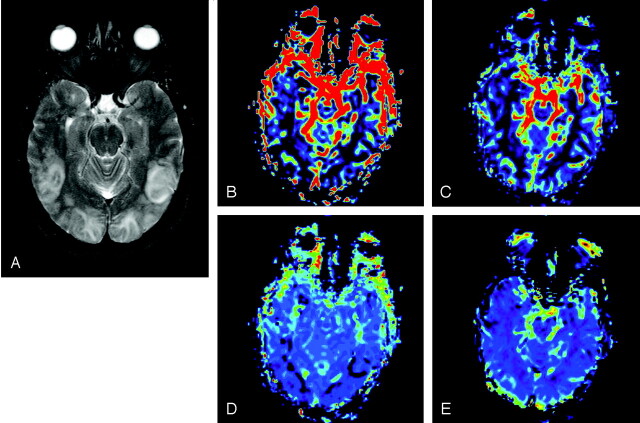
Fig 1.
MR imaging of patient 1 within 24 hours of onset of symptoms. A, FLAIR MR image demonstrates bilateral posterior edema in the cortical and subcortical posterior brain. CBV (B) CBF (C), MTT (D), and K2 (E) maps illustrate a significant decrease in CBV, CBF, and MTT posteriorly, whereas K2 values are unchanged.
Fig 2.
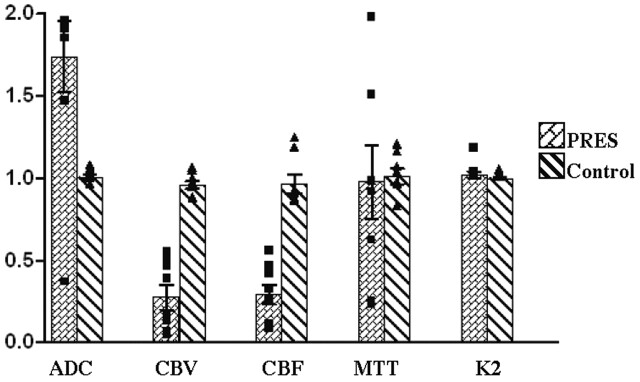
Comparison of hemodynamic variables between normal subjects and patients with PRES. Graph shows ratio of values measured in the occipital lobe (posterior) to frontal lobe (anterior). Value of 1 is when posterior equals anterior. Notice significant increases in the ADC, as well as decreases in CBV and CBF, in posterior affected areas in patients with PRES. MTT shows considerable increased variability in PRES. K2 is unchanged.
Image findings in seven of the eight patients resolved after 1-month follow-up. No permanent neurologic defects were observed. Patient 6 developed diffusion-weighted imaging evidence of infarction, but died while with PRES, as well as several confounding factors, including end-stage renal disease, sepsis, and multiorgan failure.
The normal subjects demonstrated very low intrasubject variability. Ratios of posterior to anterior values for ADC, CBV, CBF, MTT, and K2 parameters were calculated. These ratios for the normal patients were all very near unity, as expected, and are shown in (Table 2) and Figure 2.
TABLE 2:
Individual subject ratio: ratio of averaged posterior to anterior hemodynamic values
| Normal Controls: Ratio of Posterior/Anterior | ||||||
|---|---|---|---|---|---|---|
| Number | Age/Sex | ADC | CBV | CBF | MTT | K2 |
| 1 | 33/F | 1.01 | 0.86 | 0.85 | 1.14 | 1.00 |
| 2 | 23/F | 0.98 | 0.95 | 1.16 | 0.95 | 1.00 |
| 3 | 22/F | 1.06 | 0.98 | 0.91 | 0.94 | 1.03 |
| 4 | 35/M | 1.04 | 0.93 | 0.84 | 1.01 | 1.00 |
| 5 | 35/F | 1.01 | 1.02 | 1.22 | 0.81 | 0.99 |
| 6 | 46/F | 1.00 | 0.95 | 0.91 | 1.05 | 0.99 |
| 7 | 50/F | 0.94 | 1.04 | 0.89 | 1.18 | 0.99 |
| Mean/SD | 1.01 ± 0.04 | 0.96 ±0.06 | 0.97 ± 0.16 | 1.01 ± 0.13 | 1.00 ± 0.01 | |
| PRES: Ratio of Posterior/Anterior | ||||||
|---|---|---|---|---|---|---|
| 1 | 38/F | 2.16 | 0.05 | 0.23 | 0.23 | 1.02 |
| 2 | 66/F | 0.35 | 0.12 | 0.07 | 0.21 | 0.99 |
| 3 | 19/M | 1.83 | 0.45 | 0.31 | 1.49 | 0.99 |
| 4 | 44/F | 2.22 | 0.53 | 0.25 | 1.95 | 1.16 |
| 5 | 12/F | 1.88 | 0.50 | 0.54 | 0.60 | 0.99 |
| 6 | 42/F | 1.93 | 0.37 | 0.45 | 0.96 | 1.01 |
| 7 | 29/F | 2.12 | 0.03 | 0.10 | 0.90 | 1.00 |
| 8 | 23/F | 1.44 | 0.15 | 0.40 | 1.49 | 1.01 |
| Mean/SD | 1.74 ± 0.61 | 0.28 ± 0.21 | 0.29 ± 0.17 | 0.98 ± 0.63 | 1.02 ± 0.06 | |
Note.—As anticipated, averaged ratios for all values for control subjects are near 1, which indicates symmetry between anterior and posterior regions of the brain. For subjects with PRES, pertusion values represent the ratio of affected (posterior) to normal (anterior) regions within each subjects. F, female; M, male.
ADC values were increased in all but one PRES patient, who demonstrated moderately decreased values indicating cytotoxic edema. All eight PRES patients demonstrated decreased CBV and CBF values within the posterior affected regions (Table 2). Comparison of the mean ratios of posterior (affected) to anterior (unaffected) values in PRES versus normal subjects revealed a statistically significant decrease in posterior CBV and CBF measurements in PRES cases (P < .005; Figure 2).
MTT values varied. Four patients demonstrated decreased values, whereas the remaining three demonstrated increased values. On average, posterior MTT values were not significantly different from control values. In patients with PRES, however, there was marked variation among MTT values, which ranged from 0.21 to 1.95. Permeability (K2) values within posterior (affected) regions of the brain were unchanged in all eight PRES patients.
Discussion
The cause of PRES is not known, but many cases are associated with systemic hypertension, eclampsia, hypercalcemic crises, and neurotoxic or immunosuppressive substances (1, 2). Imaging of the brain reveals edema with a predilection for involvement in the occipital areas with cortical and subcortical white matter involvement (6, 7, 10). The pattern of bilateral occipital edema is highly suggestive of PRES, but differential diagnosis includes sagittal sinus thrombosis and bilateral posterior cerebral artery territory infarctions. With the widespread use of MR with diffusion-weighted imaging for evaluation of acute neurologic disease, PRES has been increasingly recognized. Usually there is not restriction of diffusion as would be seen with infarcts. This lack of restriction of diffusion has led to the belief that the edema seen with PRES is not caused by cell swelling—“cytotoxic edema”—which is thought to occur with ischemia, but must be caused by leakage of fluid from the vasculature—“vasogenic edema”.
Recent studies of PRES have focused on the role of blood flow changes and vascular endothelial dysfunction or injury (11–14). A study of four subjects by Yu et.al (11) found no change in CBV or MTT when regions affected by PRES were compared with contralateral normal appearing white matter. The sensitivity of their study may have been reduced by the method of comparison to contralateral white matter, however, because PRES tends to be fairly symmetrical from side to side, with greater posterior involvement.
Bartynski and Lister recently reviewed the literature for PRES, eclampsia and cyclosporine toxicity and found many studies with evidence of endothelial cell dysfunction, including release of endothelial cell surface molecules or subendothelial cell matrix components (12). Gorniak and Schwartz studied 68 patients with hypertensive encephalopathy and found significantly higher levels of von Willebrand’s antigen in those subjects that showed MR evidence of edema in the occipital lobes (13). They interpreted this as indicating a role for vascular endothelial injury (causing release of the von Willebrand’s antigen) in the edema seen with hypertensive encephalopathy.
Leakage of fluid from the vasculature can occur when the forces that govern fluid motion into and out of the microvasculature are out of balance. The hydrostatic pressure within the microvasculature drives fluid out of the microvasculature into the interstitial spaces. Hydrostatic pressure can be lowered by arterial vasoconstriction (thus reducing fluid flow into the interstitium) or elevated by arterial vasodilatation (thus increasing fluid flux into the interstitium). Venous constriction can also raise capillary hydrostatic pressure by increasing the vascular resistance downstream from the capillary bed.
The osmotic pressure caused by dissolved solutes in the blood within the microvasculature tends to draw fluid into the vasculature. This effect can be enhanced if there is an increase in the dissolved solutes in the blood (causing net fluid flow out of the interstitium) or diminished by an increase in permeability of the microvasculature to dissolved solutes (leading to net fluid flow into the interstitium). Thus, there are two likely mechanisms that can lead to interstitial (vasogenic) edema: one is an increase in hydrostatic pressure in the microvasculature, and the second is an increase in microvascular permeability.
The cerebral vasculature is capable of maintaining a relatively constant flow of blood to the brain tissues over a fairly broad range of systemic blood pressures. This is referred to as “CBF autoregulation.” Two competing hypotheses have been suggested for the mechanism of brain edema formation with hypertension. One is that the elevated blood pressure exceeds the autoregulatory capabilities of the circulation and leads to excessive blood flow and elevated capillary hydrostatic pressure, which causes fluid to leave the vessels and pass into the interstitial spaces. This hypothesis would seem to be supported by the usual increase of water diffusion (higher ADC) seen with PRES (7). The alternate hypothesis is that the hypertension elicits severe vasoconstriction, which overcompensates, leading to decreased perfusion and ischemia within the tissues. Recent studies of PRES by using MR perfusion imaging support this hypothesis (2, 5, 7). The presence of interstitial edema in the setting of decreased capillary hydrostatic pressure, however, is hard to explain. One possible explanation would be if the permeability of the microcirculation were increased. Changes in microvascular permeability were not reported in these studies.
The rationale for the current study was to examine these circulatory changes in more detail, in an attempt to gain a better understanding of the microcirculatory pathophysiology of PRES. We used perfusion imaging to measure CBV, CBF, MTT, and K2 (15, 16). MTT may help indicate the degree of vasoconstriction by providing information regarding transit time of contrast agents from the point of the arterial input to the tissue of interest (17). K2 is a measure of the rate of flux of contrast medium molecules from the vasculature into the interstitial space (18–21). Although K2 has been used to study permeability changes of abnormal vasculature in brain tumors (16, 22), its use in evaluating PRES has not been reported. Because it is a measure of contrast medium flux, K2 should be increased with increased vascular permeability; however, it may also be affected by changes in the microvascular surface area available for exchange. We hypothesized that K2 would be increased in affected brain areas in patients with PRES, reflecting increased microvascular permeability.
The eight patients with PRES analyzed in this study presented with typical symptoms including hypertension, renal insufficiency, and eclampsia. Conventional MR images demonstrated characteristic increased FLAIR and T2-weighted signal intensity in the occipital lobes (6–8). This hyperintense signal corresponded to areas of decreased CBV and CBF, supporting the autoregulatory arteriolar vasoconstriction hypothesis. Cytotoxic edema, (decreased ADC) was present in only one patient, who progressed to infarction, presumably because of very low CBF values (<20 mg/mL/min) (17). No change in K2 was detected in the affected areas of any of the patients.
Several factors may be involved in the overall lack of significant change in K2. First, changes in permeability may not be detected because of a lack of sensitivity with the perfusion-weighted imaging sequence or contrast agent. Because K2 reflects the permeability of gadolinium-DTPA, changes in microvasculature permeability to water may not be reflected by the larger gadolinium-DTPA molecule (18, 23). In addition, K2 is affected by both capillary permeability and capillary surface area available for exchange (19). Therefore, a decrease in the capillary surface area caused by vasoconstriction could cancel out the effects of increased capillary permeability. An additional factor that could affect sensitivity is that K2 is measured over a short time during and after the first pass of contrast medium through the brain tissue. A technique that uses a longer measurement time would have more chance of detecting a slow accumulation of contrast material in the extracellular space. The dynamic susceptibility technique we chose, however, has the advantage that it can be calculated from the same data obtained for blood flow calculation.
Finally, the number of subjects in our study is relatively small, which reduces sensitivity. PRES, however, is an unusual disease. Our analysis of this group of subjects does show a statistically significant decrease in CBF and CBV in the posterior (affected) areas. We were not able to show a significant difference in permeability, but a power analysis performed on the permeability data suggests that it would take an additional 54 subjects to have sufficient power to prove significance (if present) of the very small difference observed. In light of the low incidence of the disease, this was not feasible.
Another limitation of our study is the wide time range from onset of symptoms to imaging (from <1 day to 13 days). This could potentially limit group analysis of the hemodynamic parameters if any of the parameters changed over time. We reviewed the data for any trends related to time from symptom onset and found none. The CBV, CBF, and K2 ratios were all very tightly clustered, with similar patterns in all subjects regardless of time from symptom onset. The ADC ratios were all elevated and tightly clustered except in the one subject who died. The MTT values were quite variable but were not related to time from symptom onset.
The findings in four of the subjects raise another possible cause of edema in PRES. One potential explanation for the severe decreases in CBV and vasogenic edema in PRES could be that vasoconstriction is occurring predominately in the veins. It is possible that capillary hydrostatic pressure could be increased, even with significant arteriolar constriction, if there is greater proportional venular constriction, as is suggested by the greater decrease in CBV than CBF seen in five of the patients with PRES. If hydrostatic capillary pressure were increased by disproportionate venous constriction, vasogenic edema could occur in the absence of changes in capillary permeability. Although this hypothesis is difficult to prove, especially in regard to the measurement of venous CBV, recent work has suggested that this may be possible (20).
Conclusion
Using perfusion imaging in patients with PRES, we found decreased posterior CBF and CBV, with heterogeneous patterns of MTT. We did not detect changes in permeability by using the measure K2. We propose the interstitial type of edema seen in PRES might be caused by elevation of capillary hydrostatic pressure mediated by venous constriction.
Acknowledgments
We wish to acknowledge Massachusetts General Hospital for production of the MR perfusion task card, in particular Gregory Sorenson PhD and Thomas Benner PhD. We also thank University of North Carolina, Chapel Hill, MR technologists for their assistance in imaging subjects.
References
- 1.Kastrup O, Maschke M, Wanke I, Diener H. Posterior reversible encephalopathy syndrome due to severe hypercalcemia. J Neurol 2002;249:1563–1566 [DOI] [PubMed] [Google Scholar]
- 2.Sundgren P, Edvardson B, Holtas S. Serial investigarion of perfusion disturbances and vasogenic oedema in hypertensive encephalopathy by diffusion and perfusion weighted imaging. Neuroradiology 2002;44:299–304 [DOI] [PubMed] [Google Scholar]
- 3.Arora A, Chowdhury D, Daga M, et al. Reversible posterior leukoencephalopathy syndrome: a report of 2 cases. Neurol India 2001;49:311–313 [PubMed] [Google Scholar]
- 4.Eichler F, Wang P, Wityk R, et al. Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 2002;23:833–837 [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor M, Jackson A, Weller J. Dynamic susceptibility contrast enhanced MRI in reversible posterior leukoencephalopathy syndrome associated with haemolytic uraemic syndrome. Br J Radiol 2000;73:438–442 [DOI] [PubMed] [Google Scholar]
- 6.Hinchley J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500 [DOI] [PubMed] [Google Scholar]
- 7.Provenzale JM, Petrella JR, Cruz LC Jr, et al. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2000;22:1455–1461 [PMC free article] [PubMed] [Google Scholar]
- 8.Engelter ST, Petrella JR, Alberts MJ, Provenzale JM. Assessment of cerebral microcirculation in a patient with hypertensive encephalopathy using MR perfusion imaging. AJR Am J Roentgenol 1999;173:1491–1493 [DOI] [PubMed] [Google Scholar]
- 9.Weisskoff RM, Boxerman JL, Sorenson AG, et al. Simultaneous blood volume and permeability mapping using a single Gd-based contrast injection [Abstract]. In: Proceedings of the Society of Magnetic Resonance second meeting. San Francisco: Society of Magnetic Resonance;1994. :1:279 [Google Scholar]
- 10.Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 2000;21:1199–1206 [PMC free article] [PubMed] [Google Scholar]
- 11.Yu E, Roberts TPL, Mikulis DJ, Keller A. Physiologically specific imaging of posterior reversible encephalopathy syndrome [Abstract]. In: Proceedings of the American Society of Neuroradiology 42nd annual meeting, Seattle. Oak Brook, IL: American Society of Neuroradiology;2004. :18 [Google Scholar]
- 12.Bartynski WS, Lister J. Cyclosporin A/FK-506 neurotoxicity, preeclampsia/eclampsia and PRES syndrome: different names but similar systemic pathophysiology [abstract]. Proceedings of the American Society of Neuroradiology 42nd annual meeting, Seattle. Oak Brook, IL: American Society of Neuroradiology;2004. :86 [Google Scholar]
- 13.Gorniak RJT, Schwartz RB. Hypertensive encephalopathy: an investigation into the role of endothelial dysfunction [Abstract]. Proceedings of the American Society of Neuroradiology 42nd annual meeting, Seattle. Oak Brook, IL: American Society of Neuroradiology;2004. :82 [Google Scholar]
- 14.Gkogkas C, Klufas RA, Henderson GV, et al. Autonomic dysfuntion-induced hypertensive encephalopathy [Abstract]. Proceedings of the American Society of Neuroradiology 42nd annual meeting, Seattle. Oak Brook, IL: American Society of Neuroradiology;2004. :151 [Google Scholar]
- 15.Petrella JR, Provenzale JM. MR perfusion imaging of the brain: techniques and applications. AJR Am J Roentgenol 2000;175:207–219 [DOI] [PubMed] [Google Scholar]
- 16.Provenzale JM, Wang GR, Brenner T, et al. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 2002;178:711–716 [DOI] [PubMed] [Google Scholar]
- 17.Lin W, Lee JM, Lee YZ, et al. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke 2003;34:64–70 [DOI] [PubMed] [Google Scholar]
- 18.Maeda M, Maley JE, Crosby DC, et al. Application of contrast agents in the evaluation of stroke: conventional MR and echo-planar MR imaging. J Magn Reson Imaging 1997;7:23–28 [DOI] [PubMed] [Google Scholar]
- 19.Pintacuda G, Otting G. Identification of protein surfaces by NMR measurements with a paramagnetic Gd(III) chelate. J Am Chem Soc 2002;124:372–373 [DOI] [PubMed] [Google Scholar]
- 20.An H, Lin W, Celik A, Lee YZ. Quantitative measurements of cerebral metabolic rate of oxygen utilization using MRI: a volunteer study. NMR Biomed 2001;14:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.21. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997;7:91–101 [DOI] [PubMed] [Google Scholar]
- 22.Kiessling F, Krix M, Heilman M, et al. Comparing dynamic parameters of tumor vascularization in nude mice revealed by magnetic resonance imaging and contrast-enhancing intermittent power Doppler sonography. Invest Radiol 2003;38:516–524 [DOI] [PubMed] [Google Scholar]
- 23.Østergaard L, Hochberg F, Rabinov J, et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg 1999;90:300–305 [DOI] [PubMed] [Google Scholar]