Abstract
The liver is one of the most important organs, both in terms of the different metabolic processes (energy, lipid, ferric, uric, etc.) and of its central role in the processes of detoxification of substances of food origin or noxious substances (alcohol, drugs, antibiotics, etc.). The development of a relevant model that reproduces some of the functions of this tissue has become a challenge, in particular for human medicine. Thus, in recent years, most studies aimed at producing hepatocytes in vitro with the goal of developing hepatic 3D structures have been carried out in the human model. However, the tools and protocols developed using this unique model can also be considered to address physiological questions specific to this tissue in other species, such as the pig, chicken, and duck. Different strategies are presently being considered to carry out in vitro studies of the hepatic metabolism of these agronomic species.
Keywords: Liver organoids, Pluripotent stem cells, Mammals, Birds, Domestic species
Introduction
The liver is a multifunctional organ that is central in controlling the metabolism of carbohydrates (conversion of glucose to glycogen), lipids (production of cholesterol and associated proteins), and amino acids (by regulating their circulating levels); in the synthesis of certain essential proteins (albumin, coagulation factors, etc.); in regulating the levels of circulating iron; and in converting ammonia to urea and bile by capturing bile acids. The liver also plays a key role in the detoxification of exogenous substances, such as antibiotics, alcohol, drugs, or other toxic substances. The multiple physiological functions of this organ are mainly ensured by hepatocytes, which constitute more than 80% of the cells of the liver and are organized in lobules. Other morphotypes are present in the liver, such as cholangiocytes in the bile ducts, endothelial cells, Kupffer cells (which are considered as hepatic macrophages) and hepatic stellate cells (which are resident lipid-storing cells) [1, 2]. During development, the hepatic outline appears on the ventral side of the distal end of the anterior intestine and results from complex interactions between mesoderm and endoderm cells at the intra- and extraembryonic junction of the yolk sac. Certain growth factors, such as Fibroblast growth factor (FGF), Bone morphogenetic proteins (BMPs), WNT signaling and retinoids allow the cellular signaling that is necessary for this differentiation of the hepatic lineage during development with a rapid dynamic [3–5].
Hepatocytes are extremely polarized epithelial cells that are organized into lobules. Lobules are anatomo-physiological structures that are more or less well defined morphologically according to animal species, with large disparities observed between humans and pigs including fibrotic bridges between periportal zones [6]. The basolateral side of the lobules is irrigated by the hepatic sinusoids, which are specialized capillaries organized around a vascular structure, i.e., a central vein that is connected with the other central veins of each lobule; together, these vascular structures end up constituting the major vascular system of the liver, the vena cava hepatic. The apical surface of the hepatocytes is in greater contact with the bile ducts, which produce the bile that flows into the duodenum. Thus, this particularly vascularized assembly ensures the very important blood flow of this organ.
One of the most original and unique properties of the liver tissue is its regenerative capacity. In the event of injury, this tissue regenerates in a process that takes between 1 and 2 weeks in humans, and becomes indistinguishable from the original tissue after a few weeks. The main factors that have been identified as being involved in this regenerative process are the hepatocyte growth factor (HGF), insulin, the transforming growth factor-alpha (TGFα), the epidermal growth factor (EGF), interleukin-6 (IL-6), and norepinephrine. Some of these factors and their receptors may have redundant roles depending on the species [7]. While the participation of tissue stem cells is undeniable in this regenerative process, the nature of these cells has been the subject of numerous studies and controversies [8, 9].
Induced pluripotent stem cells, a source of hepatoblasts
The approaches that have been used to develop liver organoids are two-fold, as in many tissues. An organoid is defined as an in vitro three-dimensional structure that self-organizes from stem cells and has the capacity to self-renew and to differentiate to give rise to the different constitutive morphotypes of the tissue it aims to mimic and to reproduce at least some of its physiological functions. For liver organoids, there are protocols for obtaining spheroids/organoids from hepatic cell lines, such as HepG2 or HepaRG cells [10, 11], from biopsies or from already formed tissue [12–15]. In this first approach, the structures obtained depend on the quality of the samples, and their establishment requires an extracellular matrix and occurs over 4−8 weeks. The second approach is based on the use of pluripotent stem cells (PSCs). Adaptations of the protocols were made to allow the initial induction of those cells into a final endoderm, from which the hepatoblasts and then the hepatocyte-like cells are derived using a maturation process in the presence of various inducers and growth factors, but with a greater or lesser homogeneity in the cell types obtained [16–19]. The process that starts from PSCs is longer (10−15 weeks), but leads to a hepatic organoid structure that is more complex and closer to the functional level of liver tissue. The comparison of the two approaches revealed the advantages and disadvantages of each of the approaches [20].
Concomitantly, direct somatic reprogramming made it possible to obtain liver cells in both murine and human species using different combinations of genes, including FOXA3, HNF1A, and HNF4A [21, 22], or more complex systems, such as HNF1A, HNF4A, and HNF6 supplemented with maturation factors (i.e., ATF5, PROX1, and CEBPα) [23]. A complementary and original approach consisted in the use of a single reprogramming factor (HNF1α) in the presence of different molecules that control specific signaling pathways [24]. This strategy resulted in the production of cells with markers and several hepatocyte functions in vivo.
If the cultures were initially developed into two-dimensional adherent cells, three-dimensional culture approaches have appeared more recently to allow the production of hepatic organoids and their long-term maturation. Thus, these recently described protocols allowed the establishment of structures that are maintained over time and for some of them reproduced “mature liver properties, including serum protein production, drug metabolism and detoxifying functions, active mitochondrial bioenergetics, and regenerative and inflammatory responses” by stably expressing several highly specific markers including ALB, SERPINA1, TTR, HNF4a, etc.… [25, 26]. The different steps of getting hepatocytes from PSCs are almost similar between the 2D and 3D cultures with the additional step of producing floating embryoid bodies for the 3D approach. The further steps of endoderm induction, endoderm specification, hepatoblast induction and hepatocyte maturation are more or less identical with changes in duration between the different reported protocols [19, 25, 26]. However, differences are indeed observed between both systems with advantages and disadvantages as summarized in Table 1. These same protocols are also starting to reproduce the complexity of the liver tissue, as mentioned above. In fact, this complexity supposes that all hepatocyte functions are ultimately represented in these derived structures in vitro, including the presence of vascularization and bile ducts [27–31]. Synthesis of the recent reported protocols is available [32].
Table 1.
Comparison between 2 and 3D cultures for generating liver in vitro liver models
| 2D culture | 3D culture | |
|---|---|---|
| Morphology | Monolayer | Organized aggregates with multiple layers |
| Amplification | Easy and convenient |
More complex Stable long-term cultures |
| Differentiation |
Directed but limited on flat and surface Constrained morphogenesis |
Self-organization Feasibility of mixed 3D structures of hepatoblast, mesenchymal and vascular cells |
| Genes and Proteins | Hepatoblast and hepatocyte with embryonic phenotypes | More mature hepatocytes—adult-like phenotypes |
| Advantages |
Fast and rapid establishment Relatively inexpensive, Well adapted to high throughput capacity |
Partially mimicking the in vivo microenvironment Reproducing the apical-basal polarity in multicellular structures Control of factor gradient by microfluidic approaches |
| Disadvantages |
Flat surface Automatic apical-basal polarity not mimicking the tissue structure |
Costly and more laborious High throughput capacity to be optimized |
Such analysis can be extrapolated for other organoid models as well.
The challenge of producing the most relevant hepatic organoids in relation to the hepatic tissue is particularly important for different approaches, such as the open perspective in regenerative medicine, but also as models for physiological studies of many hepatic pathologies, including viral infections such as hepatitis [33] and metabolic disorders such as fibrosis and cirrhosis that could both be due to chronic injuries, nonalcoholic fatty liver disease (NAFLD), the most prevalent chronic liver disease leading to the non-alcoholic steatosis (NASH), affecting millions of people around the world [34–36]. In this context, generation of hepatic stellate cells from PSC has been reported to also contribute to the modeling of liver fibrosis [37]. The hepatic organoid model is also becoming a reference in toxicology, because these structures appear to be a major advancement in the predictive approach to the evaluation of the toxicity of a known molecule or in therapeutic testing [38, 39].
Applications in other species
Most of the studies and protocols published for generating organoids were performed using mouse and human models; thus, proportionally, the data available for other species, in particular species of agronomic interest, are scarce [40]. To highlight the interest of developing the hepatic organoid model in these species, it is also necessary to identify the similarities and the differences and therefore to compare the hepatic metabolisms between the different species, including humans. If the metabolic pathways are generally very close, the nutritional lever is also used in domestic animals, in particular to control carbohydrate metabolism and lipidogenesis which does not take place exclusively in the liver for mammals with fat deposits in peripheral tissues [41, 42].
Although the pig is often considered a possible alternative as an organ donor in xeno-transplantation approaches, few studies have been published on the formation of porcine hepatic organoids/spheroids [43], with rare exceptions, such as the bioartificial liver model that was developed for studying acute liver failure (ALF) that could be caused by various toxic compounds, chemicals or viruses that may induce a massive necrosis of parenchyma, responsible of ALF [44]. Conversely, the pig was also used as a test of the functionality of human liver organoids in this same ALF model [45]. However, there seems to be little use of this model in toxicological or nutritional approaches.
Another example is the production of hepatic organoids from tissue biopsies in cats, to propose a model for studying NASH [46]. As previously mentioned, modeling this disease is still a challenge [36] and even if numerous rodent models are developed as well as in vivo assays on minipigs, few in vitro assays with organoids of mammalian domestic animals were presently reported [42, 47, 48].
Various studies have also been carried out in the fish model, in particular for the generation of hepatic trout organoids in toxicological screening approaches [49–51].
All the current organoids obtained from species other than murine and human have been derived from tissue precursors, with PSCs and induced pluripotent stem cells (iPSCs) being neither described nor available in these species with the same properties as those observed in murine and human models. Recent advances in deriving PSCs in those species are summarized in the introductive chapter of this series, entitled “Organoids in domestic animals: with which stem cells?”. Therefore, despite those progresses, this obstacle of getting robust PSCs limits the development of original protocols in these species.
In avian species, the importance of lipid metabolism in the body composition, nutritional egg quality, and adaptation of birds to environmental changes such as feed composition changes [51–53], or during steatosis processes, render the liver an important tissue to be studied, as it is the major organ in lipid synthesis in contrast to mammals [41, 42]. As an example and as stated by Surugihalli et al., “the chicken liver is subjected to intense lipid burden from high rates of yolk-lipid oxidation and also from the accumulation of the yolk-derived and newly synthesized lipids from carbohydrates”. High rates of hepatic lipid oxidation and lipogenesis are also central features of non-alcoholic fatty liver disease (NAFLD) in both rodents and humans, but is associated with impaired insulin signaling, dysfunctional mitochondrial energetics and oxidative stress” [54]. Numerous studies have investigated the molecular basis of the avian hepatic metabolism by deciphering mainly the hepatic lipid metabolism and the associated genes [55–57]. In this context, organoids in avian species would be of great interest for studying the regulatory mechanisms of lipid metabolism. Furthermore, chicken has diverged from mammals more than 300 M years ago and is widely used to evaluate the level of gene conservation between species during evolution, with this conservation being a sign of a major biological role. Examples of lncRNA have been also reported for liver and adipose tissue as well [58].
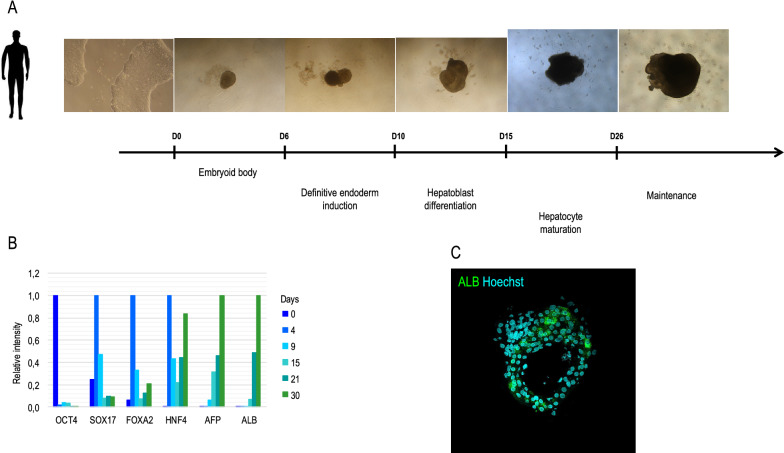
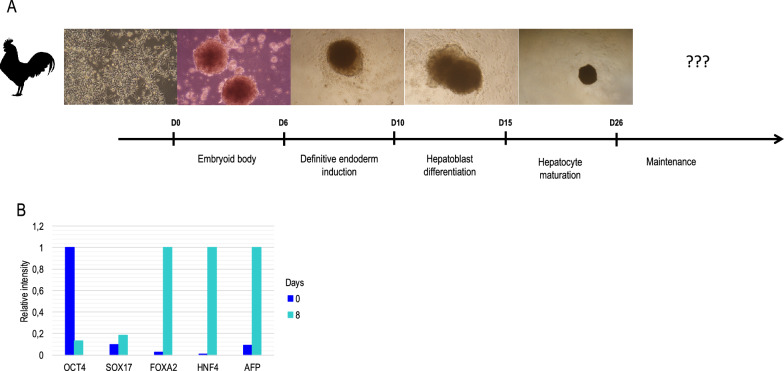
In avian species, few data are available at the level of the preparation of hepatocytes and their maintenance in culture for several days, but in each case, they were derived from adult tissue [59–62]. However, the development of organoids in avian species has not been reported. As embryonic stem cells were obtained and described in chicken [63–65], we initiated the induction of the differentiation of these PSCs in the hepatic pathway based on the human and murine models. Validated in the human model with hiPSCs, we developed a protocol that allows the establishment of hepatic organoids that produce albumin (Figure 1). In a preliminary study, this same protocol does not seem to be compatible with bird PSCs and no long-term liver organoids were obtained, even if hepatic markers were weakly detected (Figure 2). Therefore, adaptations of each step have been carried out, starting from the formation of cell aggregates that are transferred into an endodermic induction medium. These structures exhibited a pattern of proliferation and induction of markers that is specific to the engagement into this lineage. Additional work is needed.
Figure 1.
A schematic description of the generation of liver organoïds from hiPSC. A hiPSC were induced into definitive endoderm before being turned into hepatoblast and more mature hepatocyte following an adaptation of the Rashidi et al., protocol [22]. B The expression of different markers was detected by qRT-PCR and illustrates the loss of pluripotent marker (OCT4), the appearance of endoderm ones (SOX17, FOXA2) and then more specific hepatic markers (HNF4, AFP, ALB). C On mature organoïds, Albumin (ALB) is detectable by immunocytochemistry.
Figure 2.
Preliminary data with chicken liver organoïds derived from chicken embryonic stem cells (cESC). A cESC were induced into endoderm before being turned into hepatoblasts, but failed to be induced in more mature hepatocytes under present tested protocols. B the expression of different markers was detected by qRT-PCR and illustrates the loss of pluripotent marker (OCT4), the weak induction into endoderm (SOX17) and then the present failure to get more specific hepatic markers (FOXA2, HNF4, AFP) with the current conditions.
Conclusion
The development of hepatic organoids represents a real challenge for mimicking the complexity of hepatic tissue and its multiple physiological functions. Additional challenges remain to be overcome for domestic species, including the plasticity of pluripotent stem cells and the adaptation of the protocols currently described and used in the human model. We can hope that significant progress will be made in the years to come to have such in vitro models in these species.
Acknowledgements
We thank S. Lagarrigue, C. Desert and L. Lagoutte (UMR 1348, INRAE) for their valuable contributions and comments.
Abbreviations
- ALF
Acute liver failure
- BMPs
Bone Morphogenetic Factors
- EGF
Epidermal Growth Factor
- FGF
Fibroblast Growth Factor
- HGF
Hepatocyte Growth Factor
- IL-6
Interleukin-6
- iPSC
Induced pluripotent stem cell
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Non-alcoholic steato-hepatitis
- PSC
Pluripotent Stem Cell
- TGFα
Transforming Growth Factor alpha
Authors' contributions
BP wrote the manuscript with CB and GM comments. CB and GM provided the pictures in Figures 1 and 2. All authors read and approved the final manuscript.
Funding
This work was supported by grants from ANR, the project CRB-ANIM—ANR-11-INBS-0003 to BP.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27(Suppl 21):R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman MH. Stellate cells in tissue repair, inflammation, and cancer. Annu Rev Cell Dev Biol. 2018;34:333–355. doi: 10.1146/annurev-cellbio-100617-062855. [DOI] [PubMed] [Google Scholar]
- 3.Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, Ferguson A, Kimura M, Thompson WL, Wells JM, Zorn AM, Takebe T. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 2019;574:112–116. doi: 10.1038/s41586-019-1598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang LT, Tan AKY, Autio MI, Goh SH, Choo SH, Lee KL, Tan J, Pan B, Lee JJH, Lum JJ, Lim CYY, Yeo IKX, Wong CJY, Liu M, Oh JLL, Chia CPL, Loh CH, Chen A, Chen Q, Weissman IL, Loh KM, Lim B. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 2018;22:2190–2205. doi: 10.1016/j.celrep.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CY, Nichane M, Tan J, Noghabi MS, Azzola L, Ng ES, Durruthy-Durruthy J, Sebastiano V, Poellinger L, Elefanty AG, Stanley EG, Chen Q, Prabhakar S, Weissman IL, Lim B. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nykonenko A, Vávra P, Zonča P. Anatomic peculiarities of pig and human liver. Exp Clin Transplant. 2017;15:21–26. [PubMed] [Google Scholar]
- 7.López-Luque J, Fabregat I. Revisiting the liver: from development to regeneration - what we ought to know. Int J Dev Biol. 2018;62:441–451. doi: 10.1387/ijdb.170264JL. [DOI] [PubMed] [Google Scholar]
- 8.Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol. 2016;18:238–245. doi: 10.1038/ncb3309. [DOI] [PubMed] [Google Scholar]
- 10.Donato MT, Tolosa L, Gómez-Lechón MJ. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol Biol. 2015;1250:77–93. doi: 10.1007/978-1-4939-2074-7_5. [DOI] [PubMed] [Google Scholar]
- 11.Marion MJ, Hantz O, Durantel D. The HepaRG cell line: biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods Mol Biol. 2010;640:261–272. doi: 10.1007/978-1-60761-688-7_13. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Zhang G, Shen C, Uygun K, Yarmush ML, Meng Q. A novel 3D liver organoid system for elucidation of hepatic glucose metabolism. Biotechnol Bioeng. 2012;109:595–604. doi: 10.1002/bit.23349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo BK, Dietmann S, Davies SE, Praseedom RK, Lieshout R. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11:1724–1743. doi: 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- 16.Baxter MA, Rowe C, Alder J, Harrison S, Hanley KP, Park BK, Kitteringham NR, Goldring CE, Hanley NA. Generating hepatic cell lineages from pluripotent stem cells for drug toxicity screening. Stem Cell Res. 2010;5:4–22. doi: 10.1016/j.scr.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallanna SK, Duncan SA. Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol. 2013;26:1411–1413. doi: 10.1002/9780470151808.sc01g04s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters DT, Henderson CA, Warren CR, Friesen M, Xia F, Becker CE, Musunuru K, Cowan CA. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development. 2016;143:1475–1481. doi: 10.1242/dev.132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannan NR, Segeritz CP, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430–437. doi: 10.1038/nprot.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakabe K, Takebe T, Asai A. Organoid medicine in hepatology. Clin Liver Dis. 2020;15:3–8. doi: 10.1002/cld.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, Cen J, Chen X, Liu C, Hu Y, Lai D, Hu Z, Chen L, Zhang Y, Cheng X, Ma X, Pan G, Wang X, Hui L. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Du Y, Wang J, Jia J, Song N, Xiang C, Xu J, Hou Z, Su X, Liu B, Jiang T, Zhao D, Sun Y, Shu J, Guo Q, Yin M, Sun D, Lu S, Shi Y, Deng H. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14:394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Lim KT, Lee SC, Gao Y, Kim KP, Song G, An SY, Adachi K, Jang YJ, Kim J, Oh KJ, Kwak TH, Hwang SI, You JS, Ko K, Koo SH, Sharma AD, Kim JH, Hui L, Cantz T, Schöler HR, Han DW. Small molecules facilitate single factor-mediated hepatic reprogramming. Cell Rep. 2016;15:814–829. doi: 10.1016/j.celrep.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 25.Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71:970–985. doi: 10.1016/j.jhep.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Thompson WL, Takebe T. Generation of multi-cellular human liver organoids from pluripotent stem cells. Methods Cell Biol. 2020;159:47–68. doi: 10.1016/bs.mcb.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 28.Tian L, Deshmukh A, Ye Z, Jang YY. Efficient and controlled generation of 2D and 3D bile duct tissue from human pluripotent stem cell-derived spheroids. Stem Cell Rev Rep. 2016;12:500–508. doi: 10.1007/s12015-016-9657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, Phelan K, Mayhew C, Helmrath M, Takebe T, Wells J, Bezerra JA. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 2017;144:1056–1064. doi: 10.1242/dev.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashidi H, Luu NT, Alwahsh SM, Ginai M, Alhaque S, Dong H, Tomaz RA, Vernay B, Vigneswara V, Hallett JM, Chandrashekran A, Dhawan A, Vallier L, Bradley M, Callanan A, Forbes SJ, Newsome PN, Hay DC. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch Toxicol. 2018;92:3117–3129. doi: 10.1007/s00204-018-2280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsuda T, Ochiya T, Sakai Y. Generation of hepatic organoids with biliary structures. Methods Mol Biol. 2019;1905:175–185. doi: 10.1007/978-1-4939-8961-4_16. [DOI] [PubMed] [Google Scholar]
- 32.Cotovio JP, Fernandes TG. Production of human pluripotent stem cell-derived hepatic cell lineages and liver organoids: current status and potential applications. Bioengineering. 2020;7:36. doi: 10.3390/bioengineering7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortega-Prieto AM, Skelton JK, Wai SN, Large E, Lussignol M, Vizcay-Barrena G, Hughes D, Fleck RA, Thursz M, Catanese MT, Dorner M. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat Commun. 2018;9:682. doi: 10.1038/s41467-018-02969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozyra M, Johansson I, Nordling Å, Ullah S, Lauschke VM, Ingelman-Sundberg M. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci Rep. 2018;8:14297. doi: 10.1038/s41598-018-32722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D, Romeo S. Human multilineage 3D spheroids as a model of liver steatosis and fibrosis. Int J Mol Sci. 2019;20:1629. doi: 10.3390/ijms20071629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 37.Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, Smout A, El Taghdouini A, Blaya D, Rodrigo-Torres D, Graupera I, Aguilar-Bravo B, Chesne C, Najimi M, Sokal E, Lozano JJ, van Grunsven LA, Verfaillie CM, Sancho-Bru P. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 2018;23:101–113.e7. doi: 10.1016/j.stem.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Shen JX, Lauschke VM. Comprehensive evaluation of organotypic and microphysiological liver models for prediction of drug-induced liver injury. Front Pharmacol. 2019;10:1093. doi: 10.3389/fphar.2019.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauschke VM, Shafagh RZ, Hendriks DFG, Ingelman-Sundberg M. 3D primary hepatocyte culture systems for analyses of liver diseases, drug metabolism, and toxicity: Emerging culture paradigms and applications. Biotechnol J. 2019;14:e1800347. doi: 10.1002/biot.201800347. [DOI] [PubMed] [Google Scholar]
- 40.Augustyniak J, Bertero A, Coccini T, Baderna D, Buzanska L, Caloni F. Organoids are promising tools for species-specific in vitro toxicological studies. J Appl Toxicol. 2019;39:1610–1622. doi: 10.1002/jat.3815. [DOI] [PubMed] [Google Scholar]
- 41.Werner G, Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135:2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- 42.Bergen WG, Brandebourg TD. Regulation of lipid deposition in farm animals: Parallels between agriculture and human physiology. Exp Biol Med. 2016;241:1272–1280. doi: 10.1177/1535370216654996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Lee DH, Park JK, Kim SK, Kwon CH, Lee SK. Potentiality of immobilized pig hepatocyte spheroids in bioartificial liver system. Transplant Proc. 2012;44:1012–1014. doi: 10.1016/j.transproceed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Chen HS, Joo DJ, Shaheen M, Li Y, Wang Y, Yang J, Nicolas CT, Predmore K, Amiot B, Michalak G, Mounajjed T, Fidler J, Kremers WK, Nyberg SL. Randomized trial of spheroid reservoir bioartificial liver in porcine model of posthepatectomy liver failure. Hepatology. 2019;69:329–342. doi: 10.1002/hep.30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Wang J, Ren H, Liu Y, Xiang C, Li C, Lu S, Shi Y, Deng H, Shi X. Hepatic spheroids derived from human induced pluripotent stem cells in bio-artificial liver rescue porcine acute liver failure. Cell Res. 2020;30:95–97. doi: 10.1038/s41422-019-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruitwagen HS, Oosterhoff LA, Vernooij IGWH, Schrall IM, van Wolferen ME, Bannink F, Roesch C, van Uden L, Molenaar MR, Helms JB, Grinwis GCM, Verstegen MMA, van der Laan LJW, Huch M, Geijsen N, Vries RG, Clevers H, Rothuizen J, Schotanus BA, Penning LC, Spee B. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Reports. 2017;8:822–830. doi: 10.1016/j.stemcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Animal models of fibrosis in nonalcoholic steatohepatitis: do they reflect human disease? Adv Nutr. 2020;11:1696–1711. doi: 10.1093/advances/nmaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedersen HD, Galsgaard ED, Christoffersen BØ, Cirera S, Holst D, Fredholm M, Latta M. NASH-inducing diets in Göttingen minipigs. Clin Exp Hepatol. 2020;10:211–221. doi: 10.1016/j.jceh.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchea C, Owen SF, Chipman JK. Functional xenobiotic metabolism and efflux transporters in trout hepatocyte spheroid cultures. Toxicol Res. 2015;4:494–507. doi: 10.1039/c4tx00160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron MG, Mintram KS, Owen SF, Hetheridge MJ, Moody AJ, Purcell WM, Jackson SK, Jha AN. Pharmaceutical metabolism in fish: using a 3-D hepatic in vitro model to assess clearance. PLoS One. 2017;12:e0168837. doi: 10.1371/journal.pone.0168837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammel T, Tsoukatou G, Jellinek J, Sturve J. Development of three-dimensional (3D) spheroid cultures of the continuous rainbow trout liver cell line RTL-W1. Ecotoxicol Environ Saf. 2019;167:250–258. doi: 10.1016/j.ecoenv.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Desert C, Baéza E, Aite M, Boutin M, Le Cam A, Montfort J, Houee-Bigot M, Blum Y, Roux PF, Hennequet-Antier C, Berri C, Metayer-Coustard S, Collin A, Allais S, Le Bihan E, Causeur D, Gondret F, Duclos MJ, Lagarrigue S. Multi-tissue transcriptomic study reveals the main role of liver in the chicken adaptive response to a switch in dietary energy source through the transcriptional regulation of lipogenesis. BMC Genomics. 2018;19:187. doi: 10.1186/s12864-018-4520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desert C, Duclos MJ, Blavy P, Lecerf F, Moreews F, Klopp C, Aubry M, Herault F, Le Roy P, Berri C, Douaire M, Diot C, Lagarrigue S. Transcriptome profiling of the feeding-to-fasting transition in chicken liver. BMC Genomics. 2008;9:611. doi: 10.1186/1471-2164-9-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surugihalli C, Porter TE, Chan A, Farley LS, Maguire M, Zhang C, Kattapuram N, Muyyarikkandy MS, Liu HC, Sunny NE. Hepatic mitochondrial oxidative metabolism and lipogenesis synergistically adapt to mediate healthy embryonic-to-neonatal transition in chicken. Sci Rep. 2019;9:20167. doi: 10.1038/s41598-019-56715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riegler B, Besenboeck C, Bauer R, Nimpf J, Schneider WJ. Enzymes involved in hepatic acylglycerol metabolism in the chicken. Biochem Biophys Res Commun. 2011;406:257–261. doi: 10.1016/j.bbrc.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 56.Massimino W, Davail S, Secula A, Andrieux C, Bernadet MD, Pioche T, Ricaud K, Gontier K, Morisson M, Collin A, Panserat S, Houssier M. Ontogeny of hepatic metabolism in mule ducks highlights different gene expression profiles between carbohydrate and lipid metabolic pathways. BMC Genomics. 2020;21:742. doi: 10.1186/s12864-020-07093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tavernier A, Davail S, Houssier M, Bernadet MD, Ricaud K, Gontier K. Inter genotype differences in expression of genes involved in glucose metabolism in the establishment of hepatic steatosis in Muscovy, Pekin and mule ducks. Mol Biol Rep. 2020;47:1527–1533. doi: 10.1007/s11033-019-05182-y. [DOI] [PubMed] [Google Scholar]
- 58.Muret K, Désert C, Lagoutte L, Boutin M, Gondret F, Zerjal T, Lagarrigue S. Long noncoding RNAs in lipid metabolism: literature review and conservation analysis across species. BMC Genomics. 2019;20:882. doi: 10.1186/s12864-019-6093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Hea EK, Leveille GA. Lipid biosynthesis and transport in the domestic chick (Gallus domesticus) Comp Biochem Physiol. 1969;30:149–159. doi: 10.1016/0010-406x(69)91309-7. [DOI] [PubMed] [Google Scholar]
- 60.Fraslin JM, Touquette L, Douaire M, Menezo Y, Guillemot JC, Mallard J. Isolation and long-term maintenance of differentiated adult chicken hepatocytes in primary culture. Vitro Cell Dev Biol. 1992;28A:615–620. doi: 10.1007/BF02631036. [DOI] [PubMed] [Google Scholar]
- 61.Fujii M, Yoshino I, Suzuki M, Higuchi T, Mukai S, Aoki T, Fukunaga T, Sugimoto Y, Inoue Y, Kusuda J, Saheki T, Sato M, Hayashi S, Tamaki M, Sugano T. Primary culture of chicken hepatocytes in serum-free medium (pH 7.8) secreted albumin and transferrin for a long period in free gas exchange with atmosphere. Int J Biochem Cell Biol. 1996;28:1381–1391. doi: 10.1016/s1357-2725(96)00076-3. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki K, Kitaguchi Y, Fukuda T, Aoyagi Y. Ascorbic acid supplementation to primary culture of chicken hepatocytes with non-serum medium. Int J Biochem Cell Biol. 2000;32:967–973. doi: 10.1016/s1357-2725(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 63.Yamanaka N, Kitani H, Mikami O, Nakajima Y, Miura K. Serum-free culture of adult chicken hepatocytes; morphological and biochemical characterisation. Res Vet Sci. 1997;62:233–237. doi: 10.1016/s0034-5288(97)90196-x. [DOI] [PubMed] [Google Scholar]
- 64.Pain B, Clark ME, Shen M, Nakazawa H, Sakurai M, Samarut J, Etches RJ. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development. 1996;122:2339–2348. doi: 10.1242/dev.122.8.2339. [DOI] [PubMed] [Google Scholar]
- 65.Aubel P, Pain B. Chicken embryonic stem cells: establishment and characterization. Methods Mol Biol. 2013;1074:137–150. doi: 10.1007/978-1-62703-628-3_11. [DOI] [PubMed] [Google Scholar]