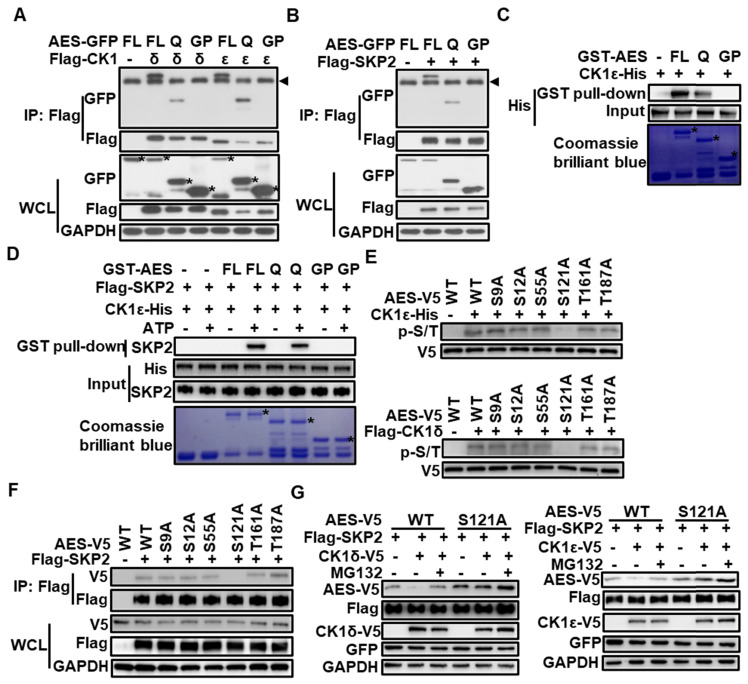
Figure 5.
CK1δ/ε phosphorylates AES at Ser121 to regulate the interaction of SKP2 with AES and the SKP2-mediated degradation of AES. (A) HEK293T cells were transfected with expression plasmids encoding GFP tagged wild-type AES, and its C- or N-terminal truncation mutations (FL, full length; Q, Q domain; GP, GP domain) along with Flag-CK1δ or Flag-CK1ε vector, respectively. Cell lysates were immunoprecipitated with anti-Flag M2 beads. Immunoblot analysis was used to detect the interaction of CK1 with AES and its mutants. The arrow head represents the IgG heavy chain. The asterisks represent full length, Q domain or GP domain of AES. (B) Similar to (A) except that Flag-SKP2 plasmid, not Flag-CK1δ or Flag-CK1ε plasmids, was transfected into HEK293T cells. The arrow head represents the IgG heavy chain. (C) In vitro GST pulldown assay was performed using purified GST-AES fragments and CK1ε-His from E. coli. GST fusion proteins were shown by Coomassie brilliant blue staining and GST was used as a negative control. CK1ε-His was detected by immunoblotting using anti-His antibody. The asterisk represents the GST fusion proteins. (D) In vitro GST pulldown assay was performed. GST-AES fragments and CK1ε-His were purified from E. coli. Flag-SKP2 was purified from HEK293T cells transfected with expression plasmid encoding Flag-SKP2. ATP (1 mM) was added as indicated. GST proteins were shown by Coomassie brilliant blue staining and GST was used as a negative control. CK1ε-His and Flag-SKP2 was detected by immunoblotting using anti-His-Tag and anti-SKP2 antibody, respectively. The asterisk represents the GST fusion proteins. (E) V5-tagged wild-type AES (WT) and its point mutants were immunoprecipitated from transfected HEK293T cells with anti-V5 agarose beads. After λ-PPase treatment, immunoprecipitated proteins were subjected to reaction with CK1ε-His purified from E. coli or Flag-CK1δ purified from HEK293T cells transfected with Flag-CK1δ expression vector in the presence of 1 mM ATP. The phosphorylated AES was detected by immunoblotting using anti-phospho-Ser/Thr (p-S/T) antibody. (F) HEK293T cells were transfected with expression vectors for V5-tagged wild-type AES (WT) and its point mutants along with empty vector or Flag-SKP2 plasmid. Cell lysates were subjected to immunoprecipitation with anti-Flag M2 beads. Immunoblot analysis was used to detect the interaction of Flag-SKP2 with AES or its point mutants. (G) HEK293T cells were transfected with the expression plasmids for AES or its S121A mutant together with Flag-SKP2 and CK1ε-V5 or CK1δ-V5 plasmids. The plasmid pEGFP-N1 was used to monitor transfection efficiency. Cell lysates were subjected to immunoblotting with the indicated antibodies. As indicated, cells were treated with 10 μM MG132 for 6 h before harvesting.